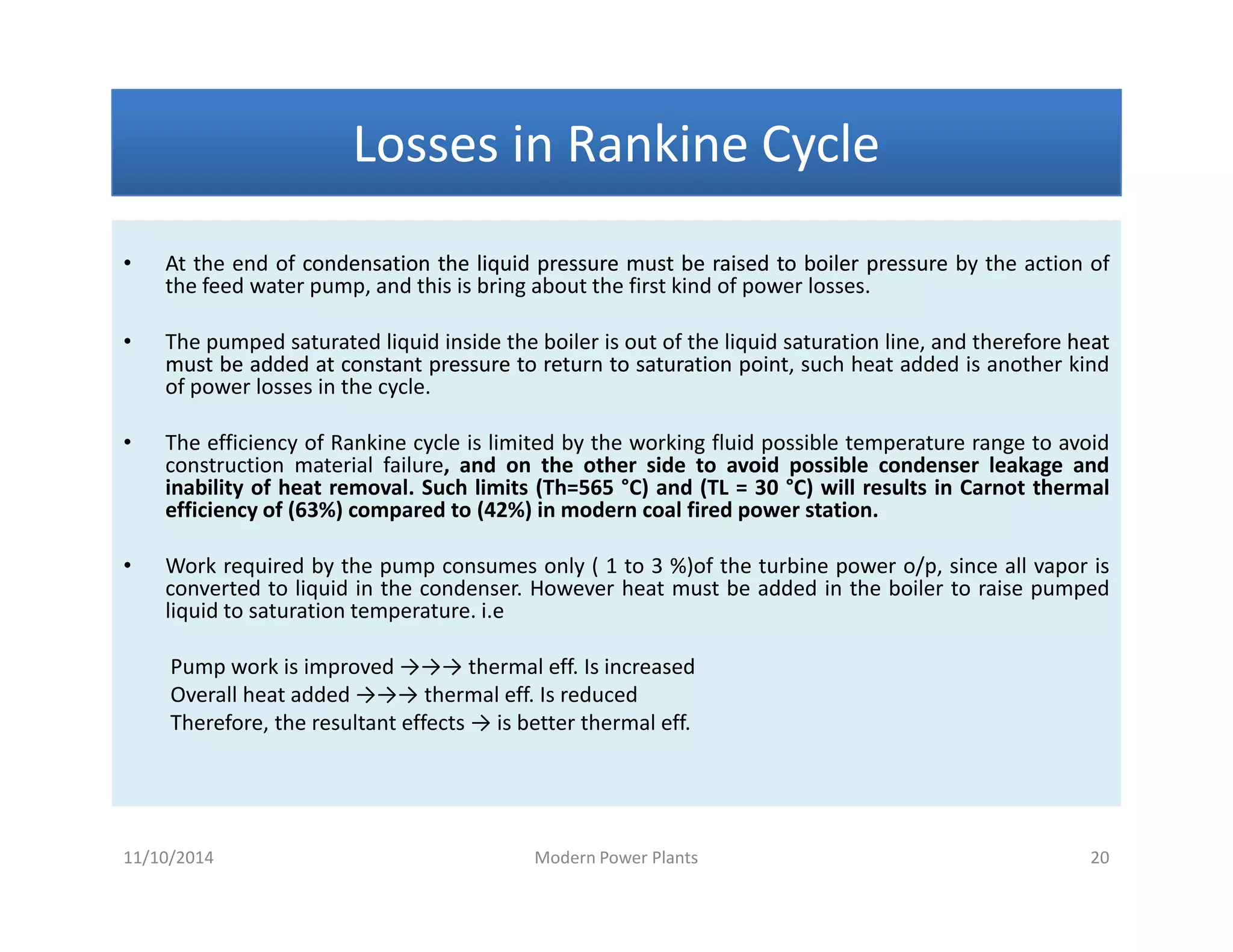
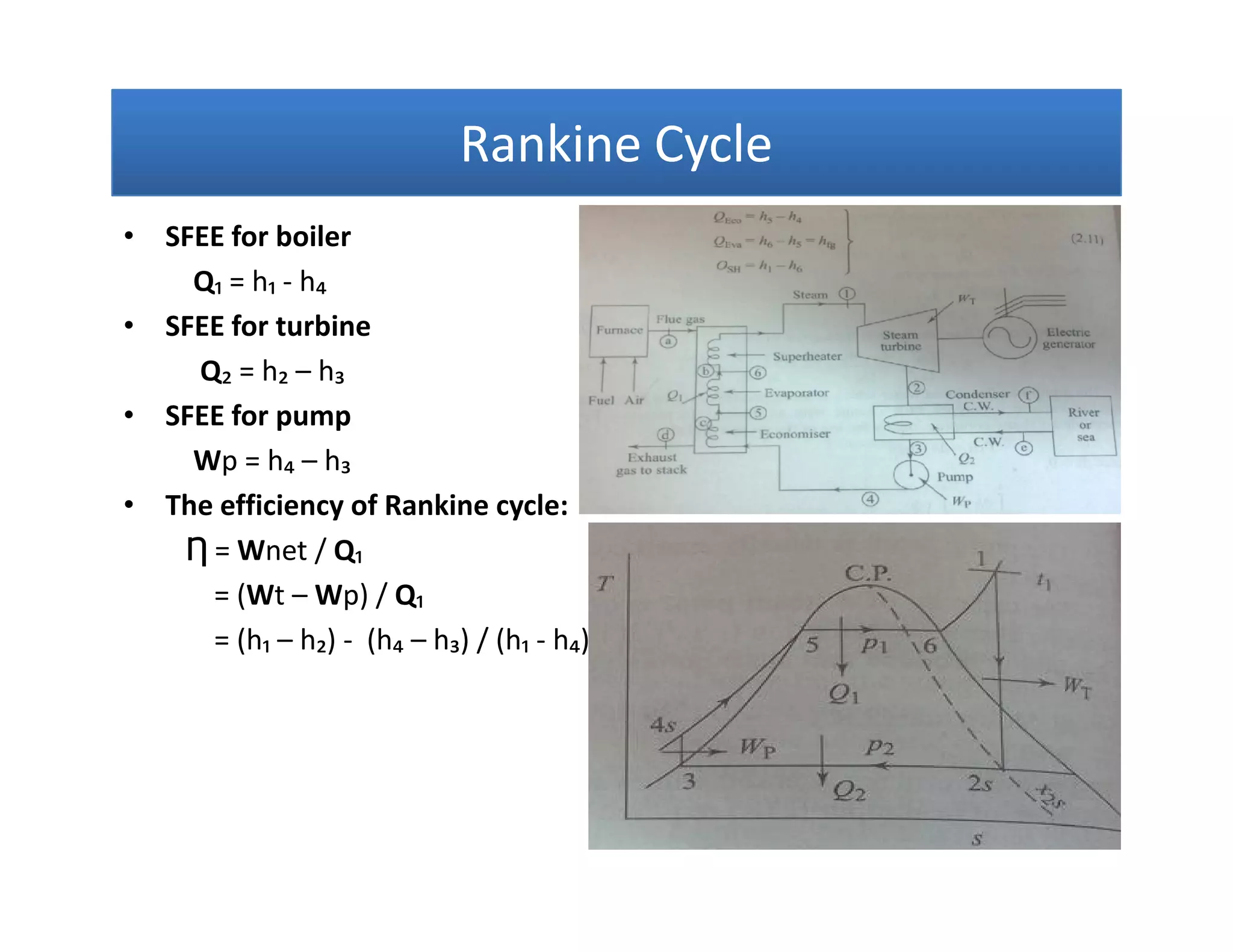
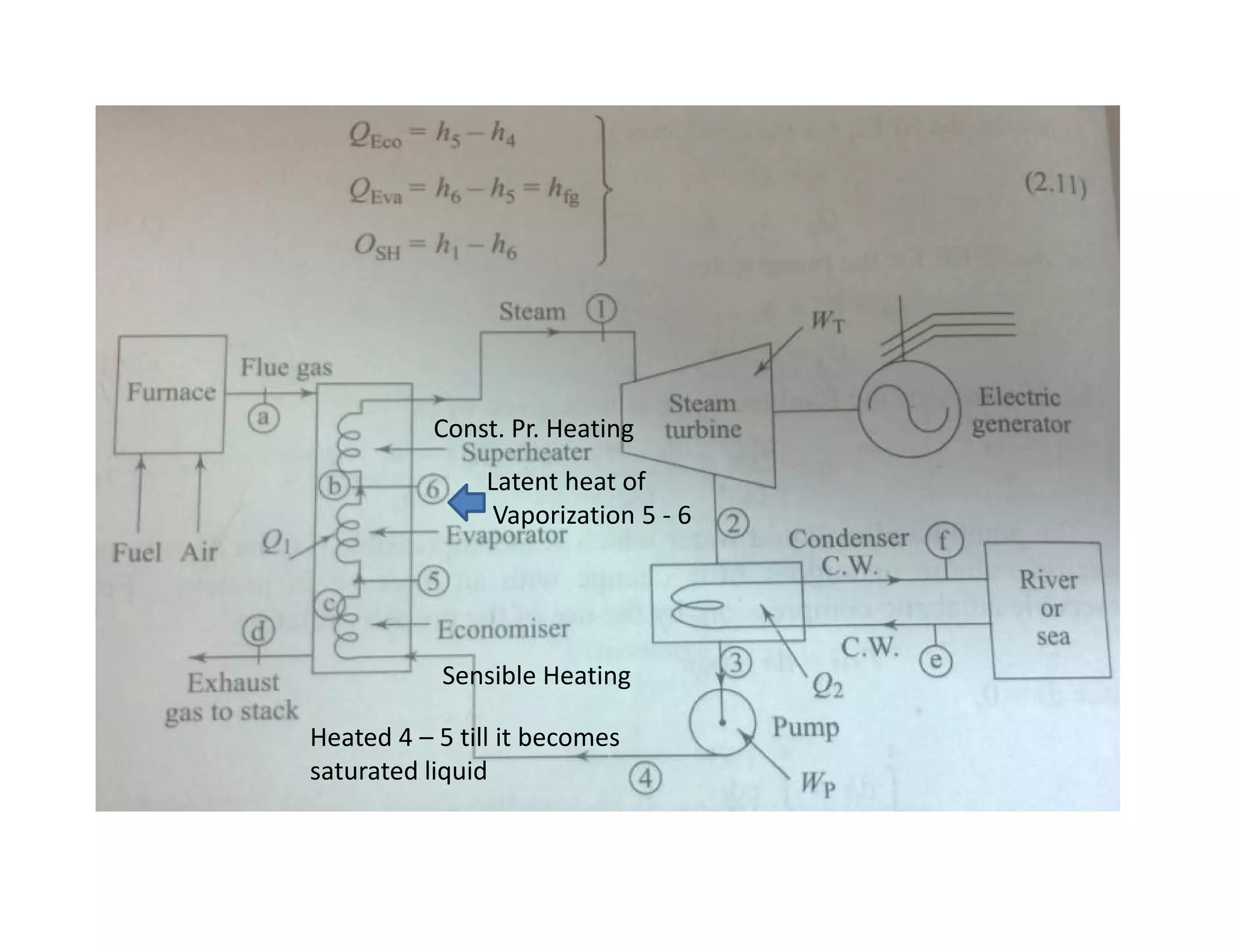
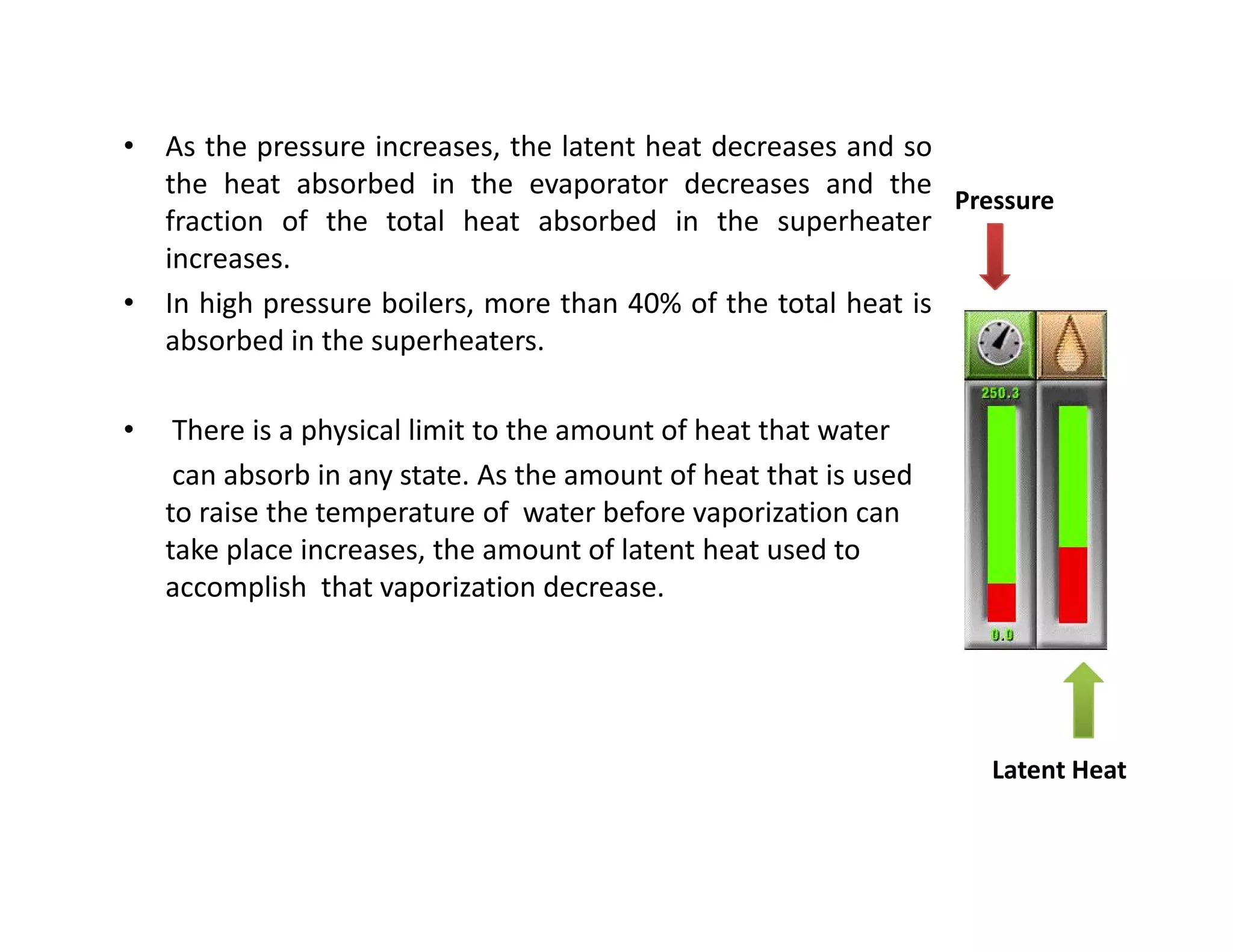
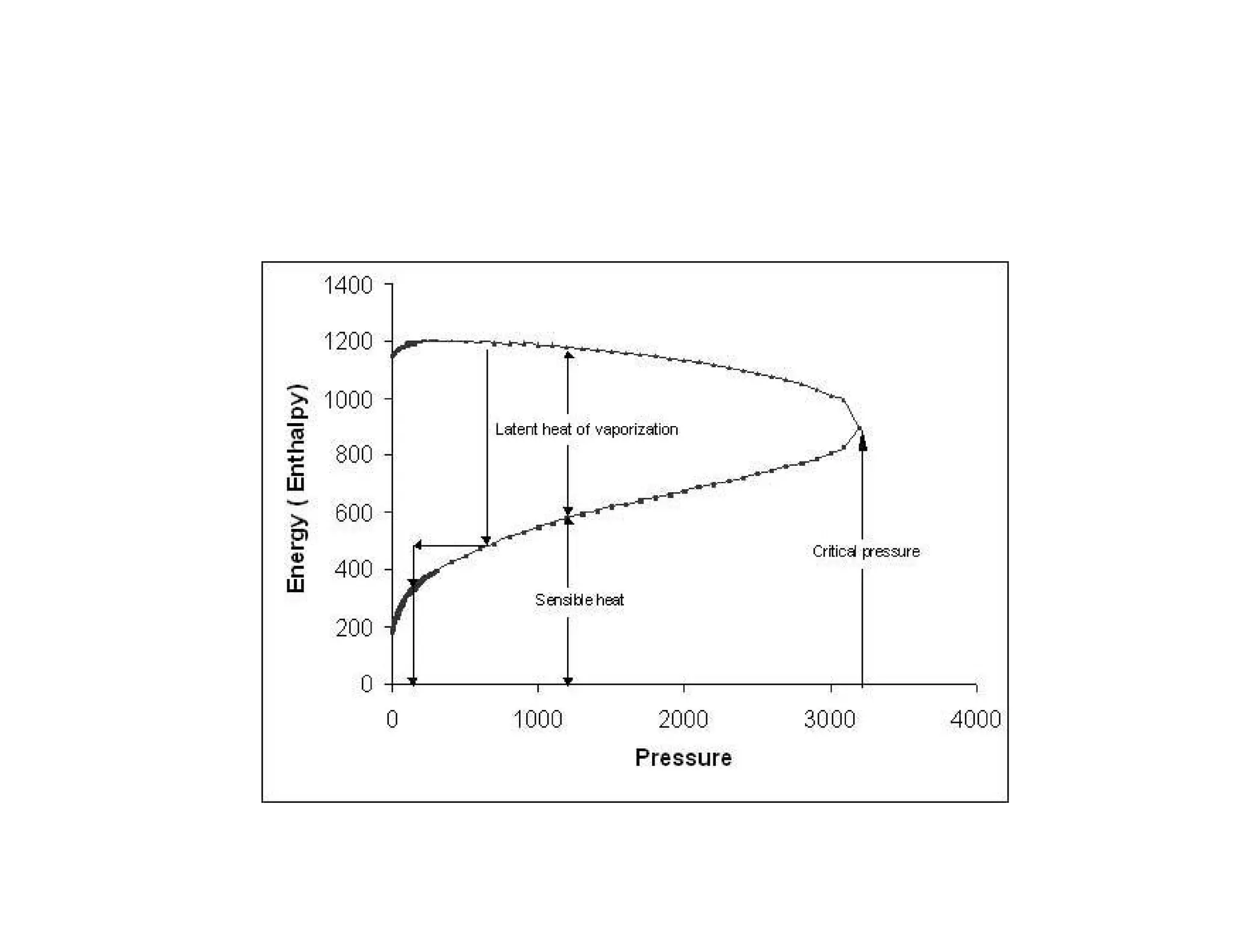
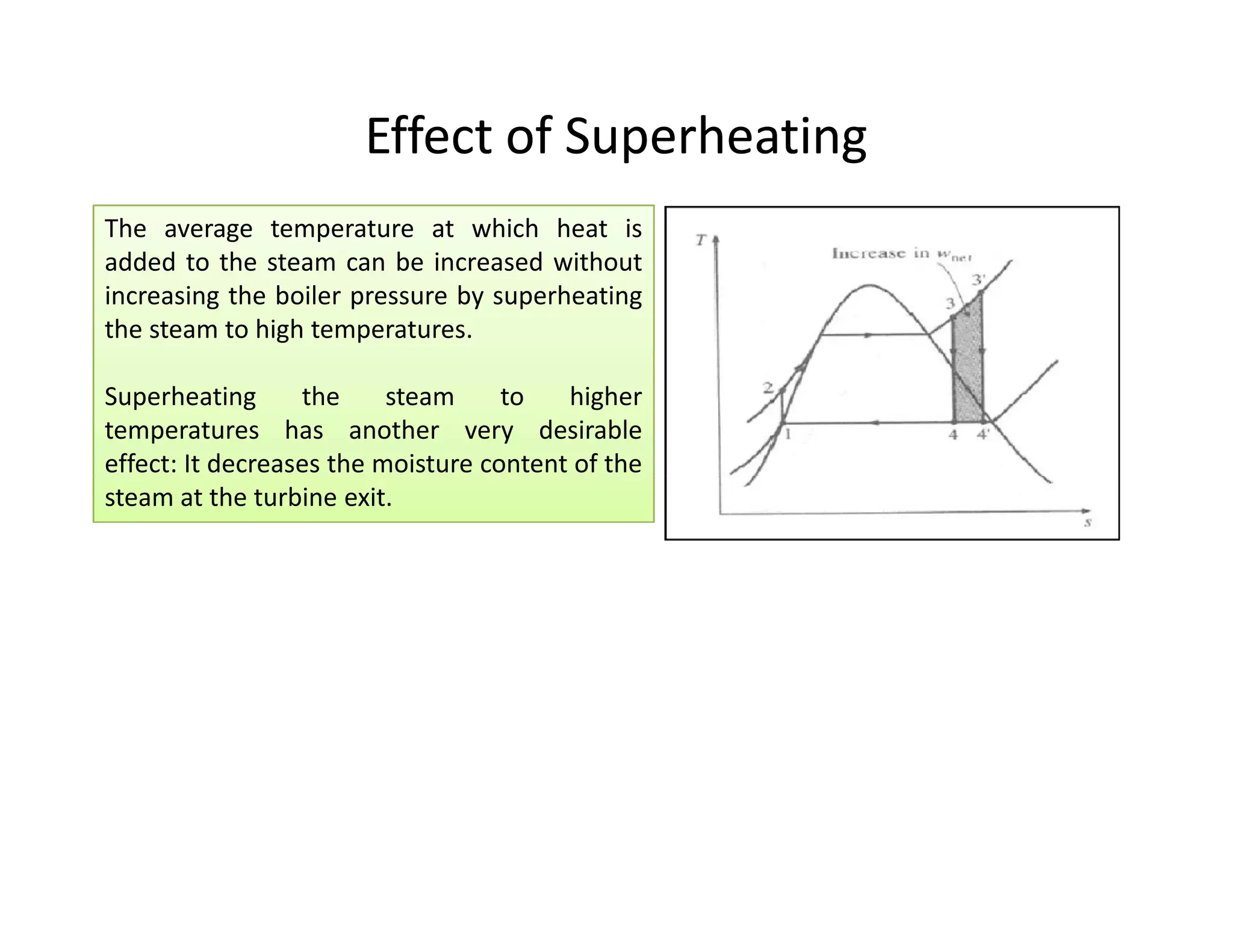
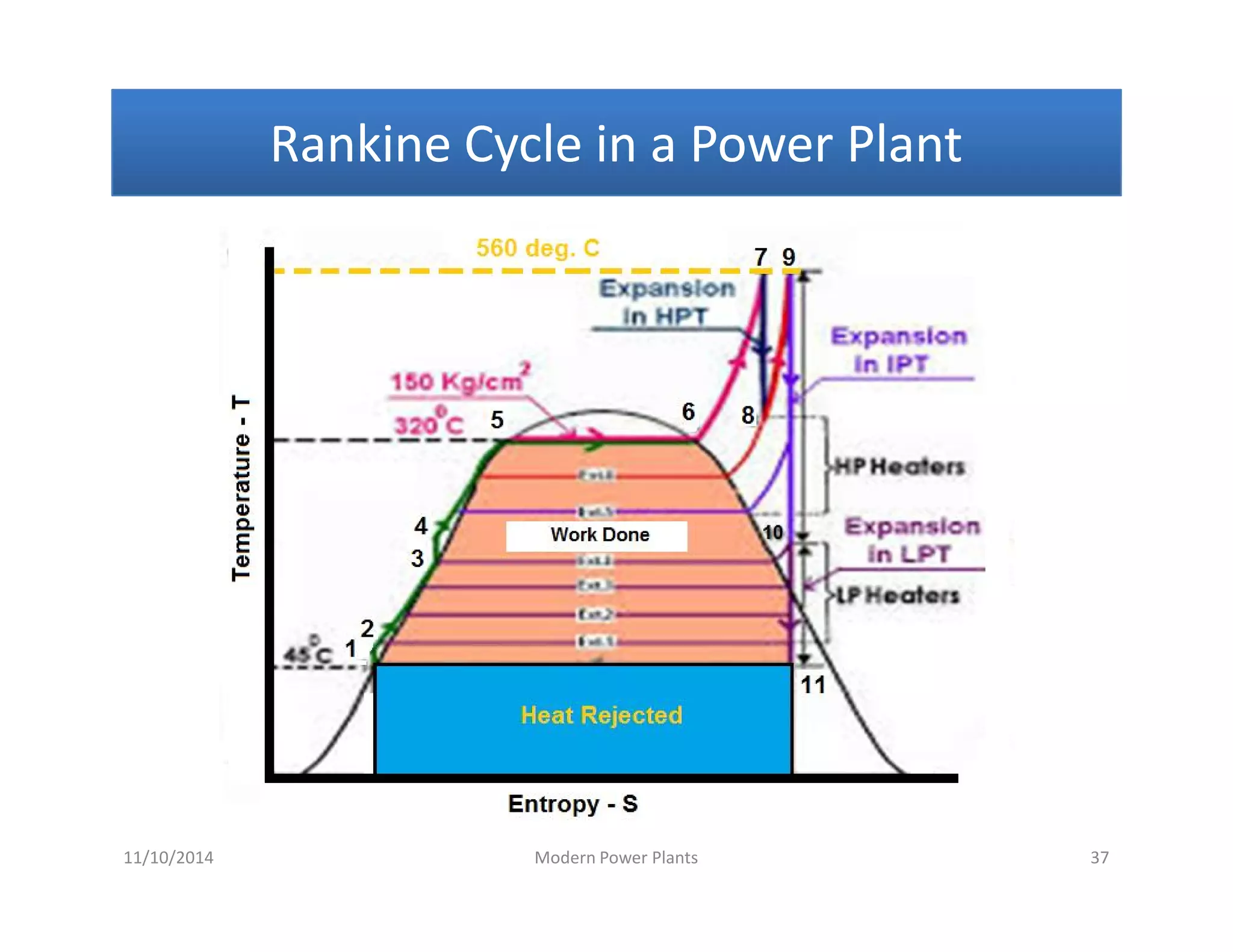
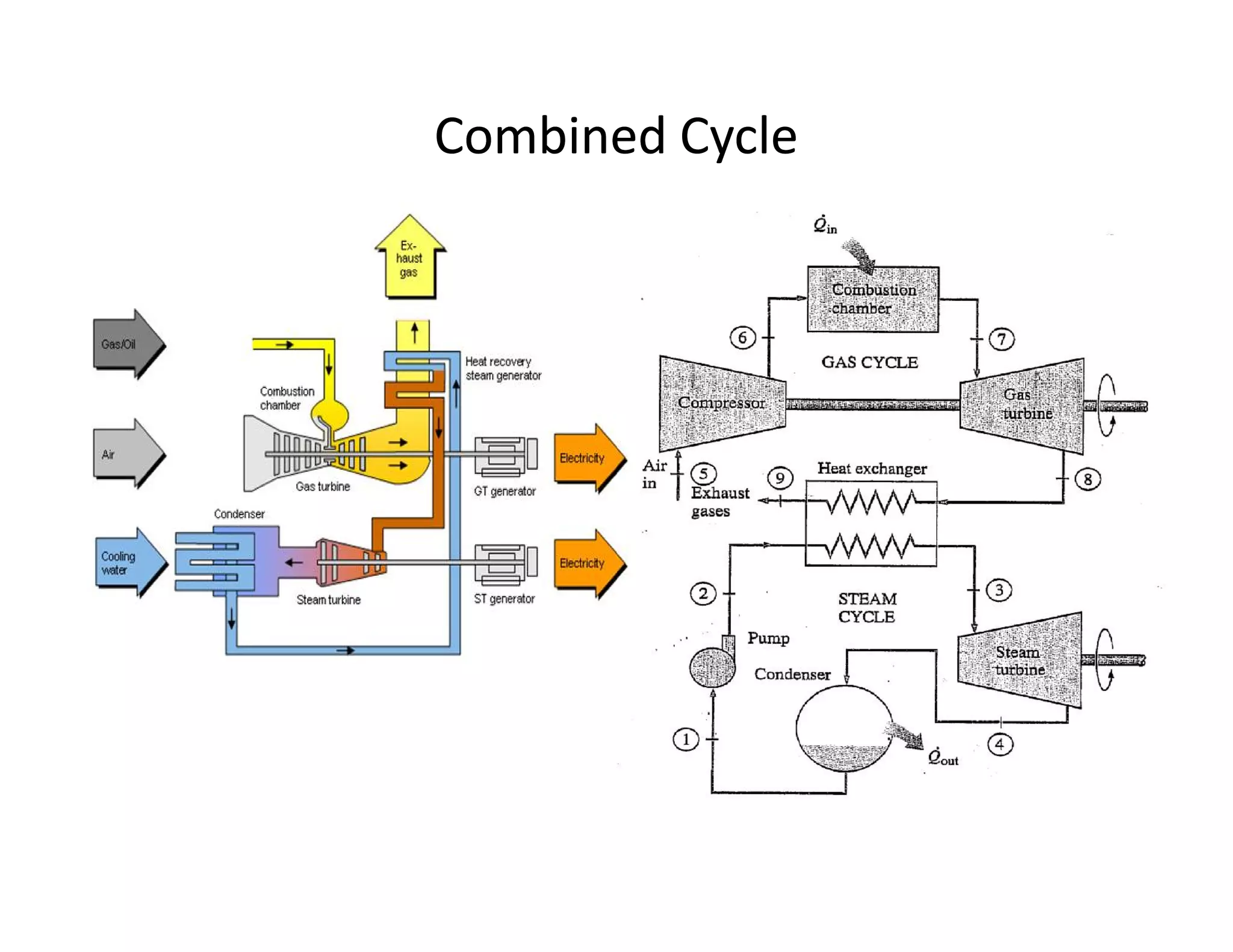
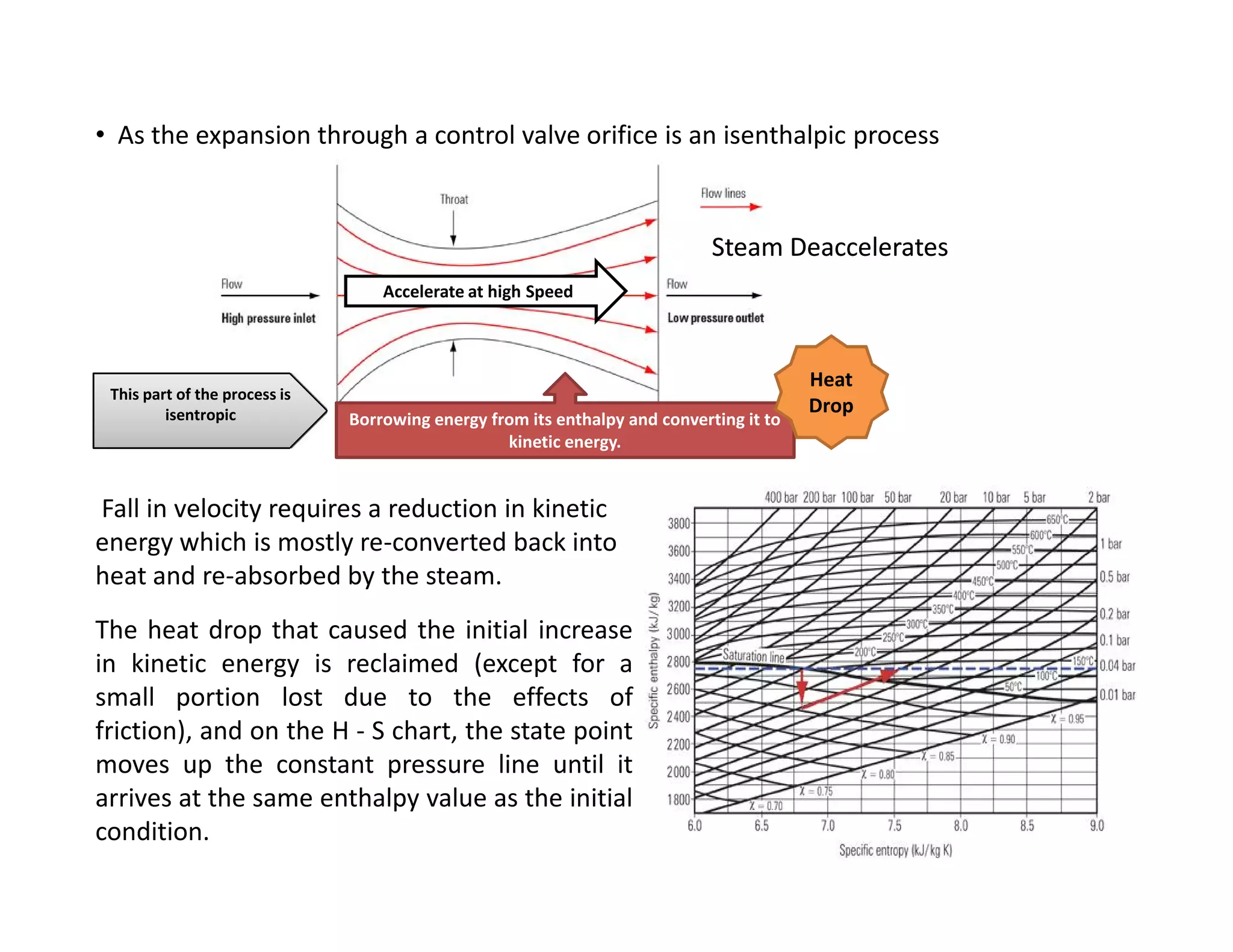
1) The document discusses different types of steam including wet steam, dry steam, and superheated steam.
2) It explains how to construct a temperature entropy diagram for steam by starting at 0°C and adding small amounts of energy to increase the temperature.
3) The diagram shows the saturated water line and saturated steam line, with the horizontal evaporation line in between where heat is added at a constant temperature during boiling.





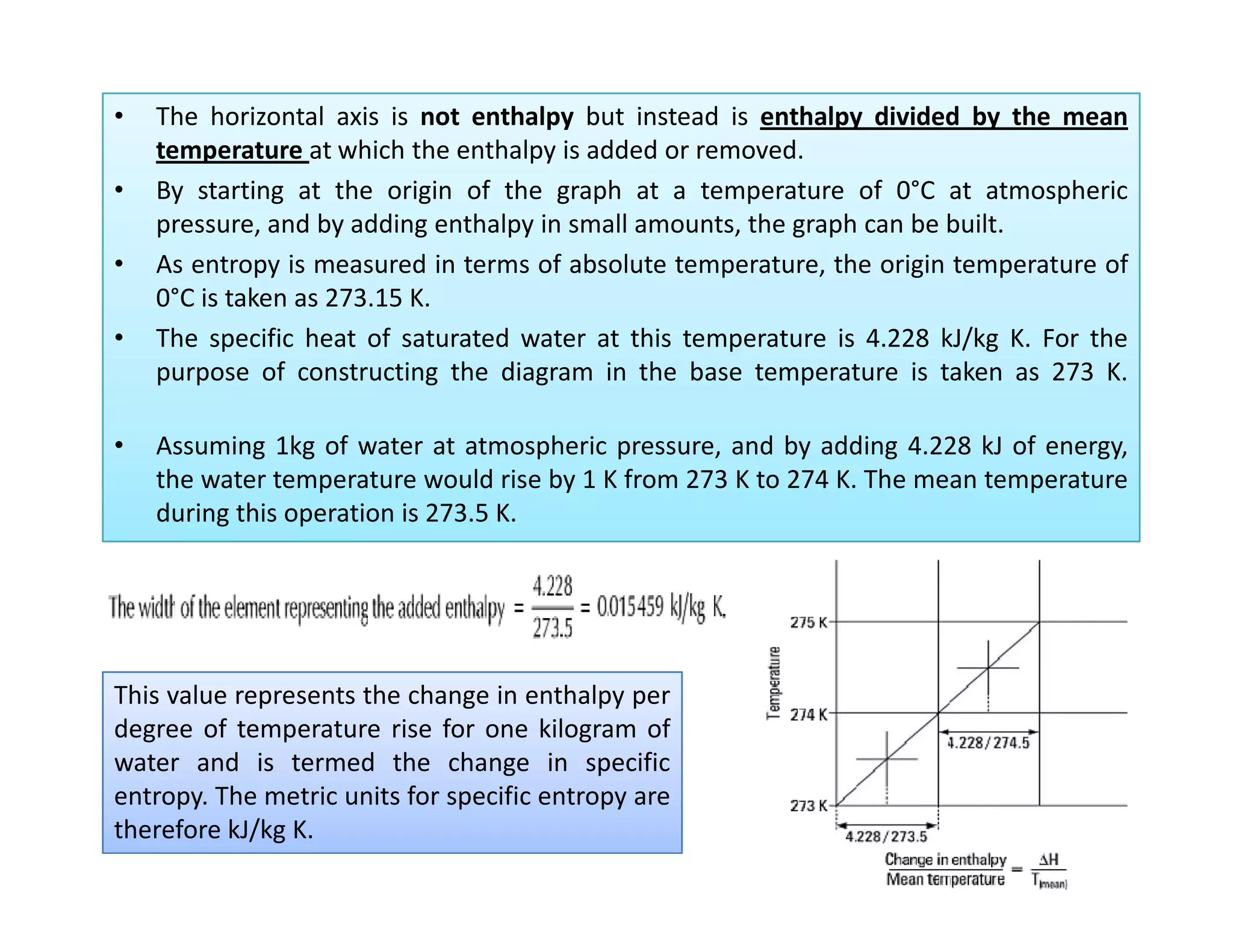



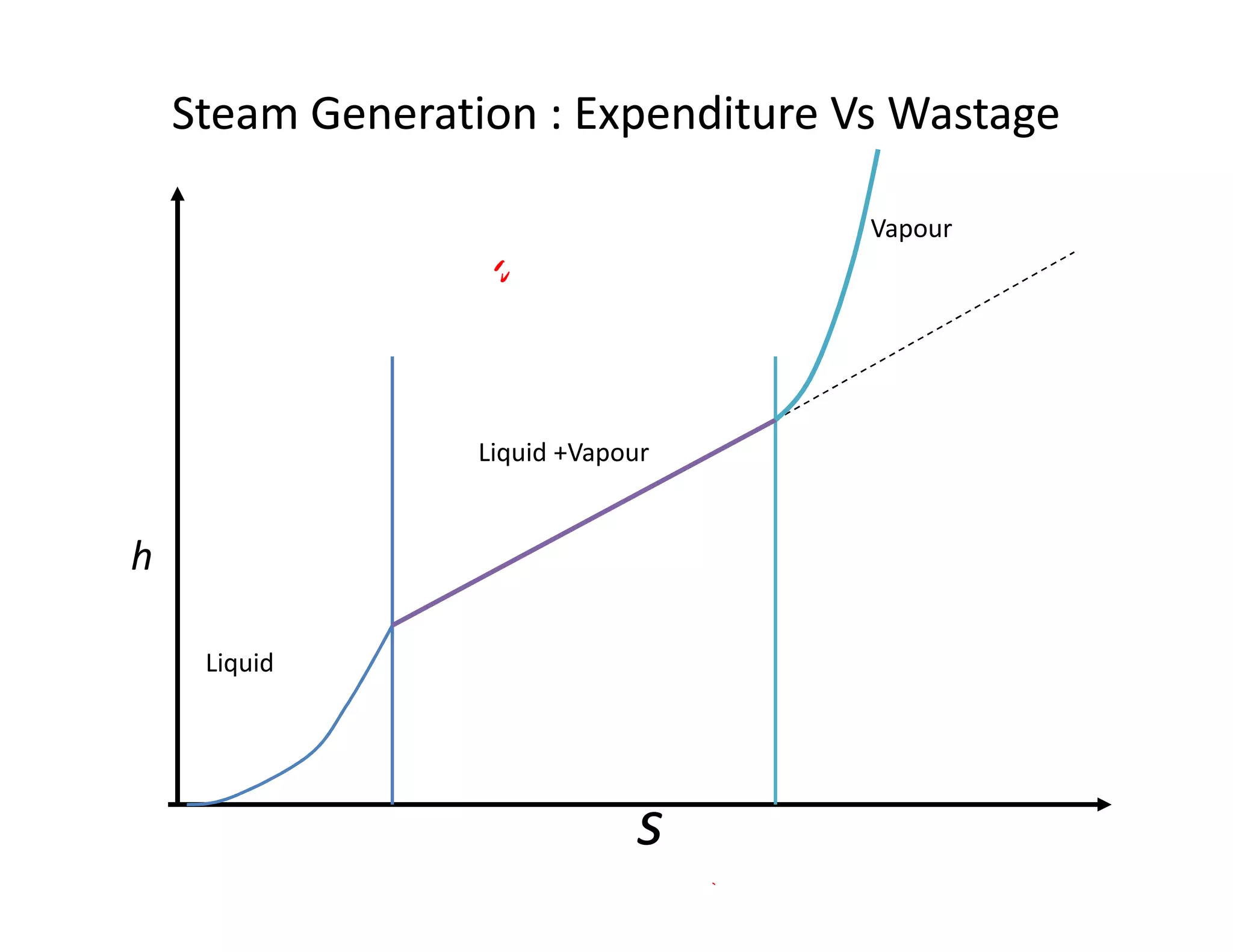

![Introduction to Rankine cycleIntroduction to Rankine cycleIntroduction to Rankine cycleIntroduction to Rankine cycle
• It is difficult if not impossible, to maintainmaintain perfectperfect constantconstant temperaturetemperature
heatheat additionaddition andand heatheat rejectionrejection..
• By usingusing waterwater asas thethe workingworking fluid,fluid, andand consideringconsidering thethe latentlatent heatheat
conceptconcept can closely resemble the theoreticaltheoretical CarnotCarnot cyclecycle.
•• Rankine cycle is a waterRankine cycle is a water--vapor cycle that describe the operation of steamvapor cycle that describe the operation of steam
power generation system in it’s thermodynamic aspects.power generation system in it’s thermodynamic aspects.
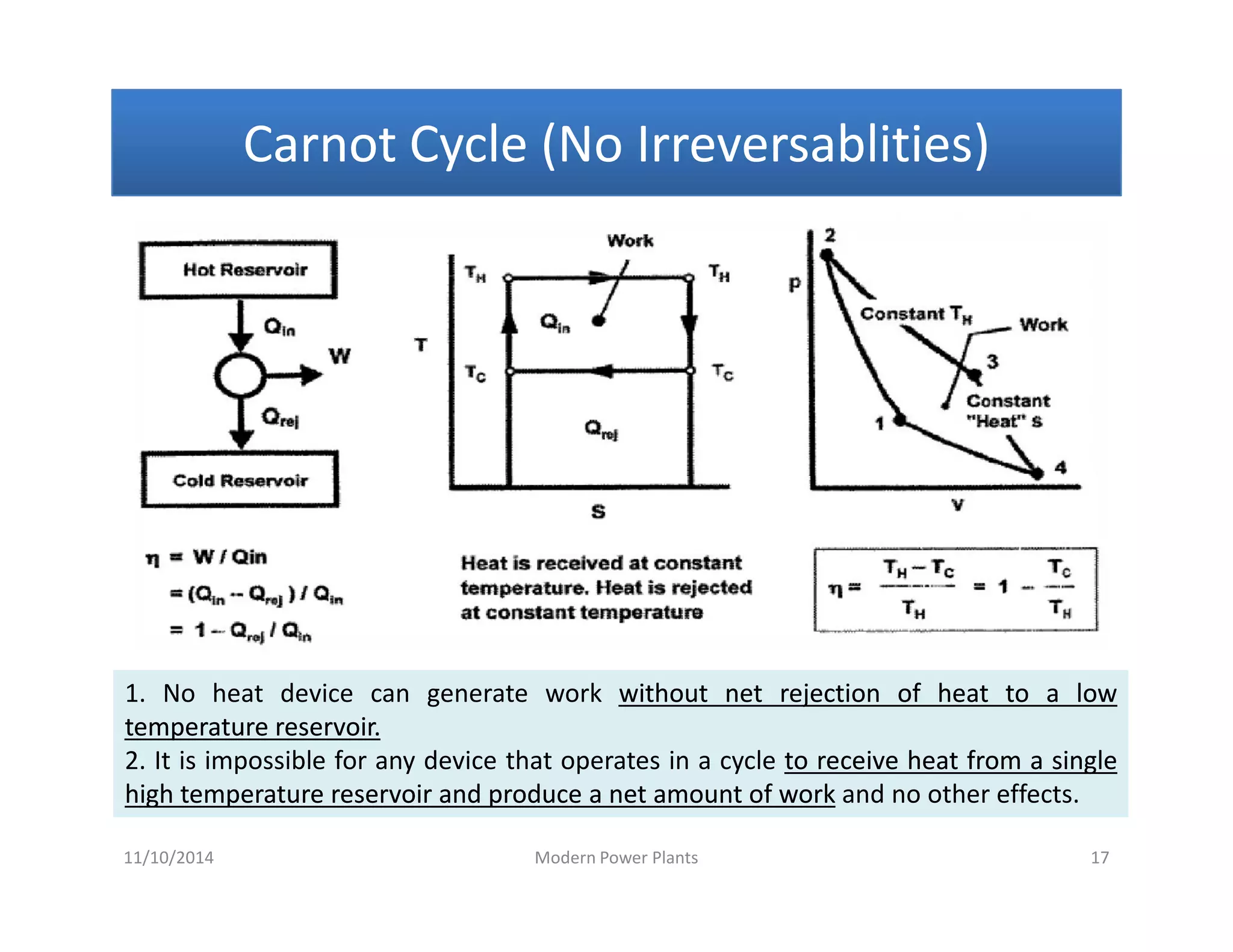
• Rankine cycle is also referred to as a “practical Carnot cycle”“practical Carnot cycle” due to :
1. The TThe T--s diagram resembless diagram resembles the Carnot cycle.
2. Heat addition in the boiler and heat rejection in the condenser takes
place :
i– Isothermally in Carnot[ ΔT = 0 ]
ii –Isobarically in Rankine[ ΔP = 0 ]
3. Steam is converted in the condenser to saturated liquidsaturated liquid
• It is difficult if not impossible, to maintainmaintain perfectperfect constantconstant temperaturetemperature
heatheat additionaddition andand heatheat rejectionrejection..
• By usingusing waterwater asas thethe workingworking fluid,fluid, andand consideringconsidering thethe latentlatent heatheat
conceptconcept can closely resemble the theoreticaltheoretical CarnotCarnot cyclecycle.
•• Rankine cycle is a waterRankine cycle is a water--vapor cycle that describe the operation of steamvapor cycle that describe the operation of steam
power generation system in it’s thermodynamic aspects.power generation system in it’s thermodynamic aspects.
• Rankine cycle is also referred to as a “practical Carnot cycle”“practical Carnot cycle” due to :
1. The TThe T--s diagram resembless diagram resembles the Carnot cycle.
2. Heat addition in the boiler and heat rejection in the condenser takes
place :
i– Isothermally in Carnot[ ΔT = 0 ]
ii –Isobarically in Rankine[ ΔP = 0 ]
3. Steam is converted in the condenser to saturated liquidsaturated liquid
11/10/2014 Modern Power Plants 18](https://image.slidesharecdn.com/analysisofsteamcycles-170121172614/75/Analysis-of-Steam-Cycles-18-2048.jpg)