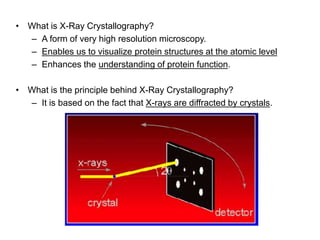
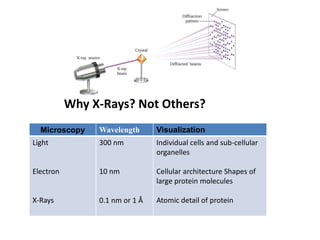
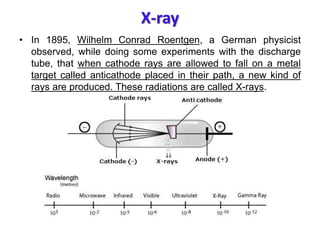
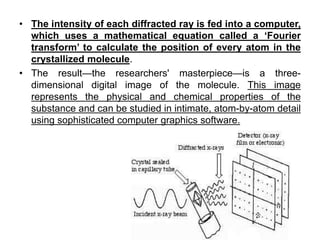
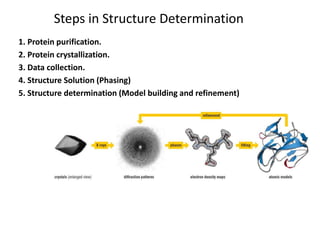
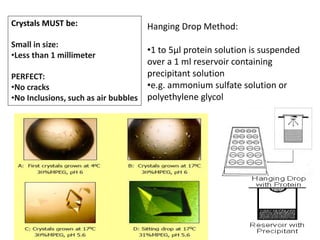
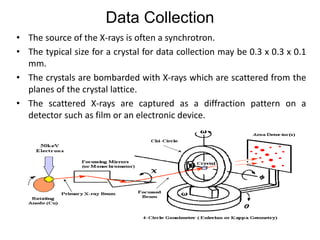
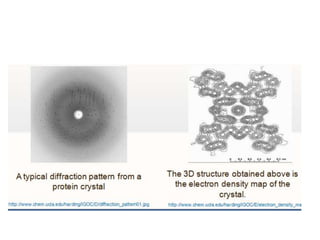
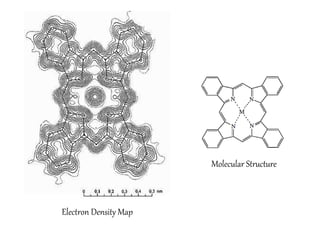
X-ray crystallography is a technique used to determine the atomic and molecular structure of crystals. It works by firing X-rays at a crystal and analyzing the diffracted rays. This allows researchers to construct a 3D model of the density of electrons within the crystal, revealing where atoms are located. Well-ordered protein crystals are required, as X-ray scattering from a single molecule would be too weak. Researchers grow crystals and collect diffraction data, which are then used to calculate atomic positions via Fourier transforms. This technique has determined over 85% of known protein structures and is invaluable for understanding functions at the molecular level.