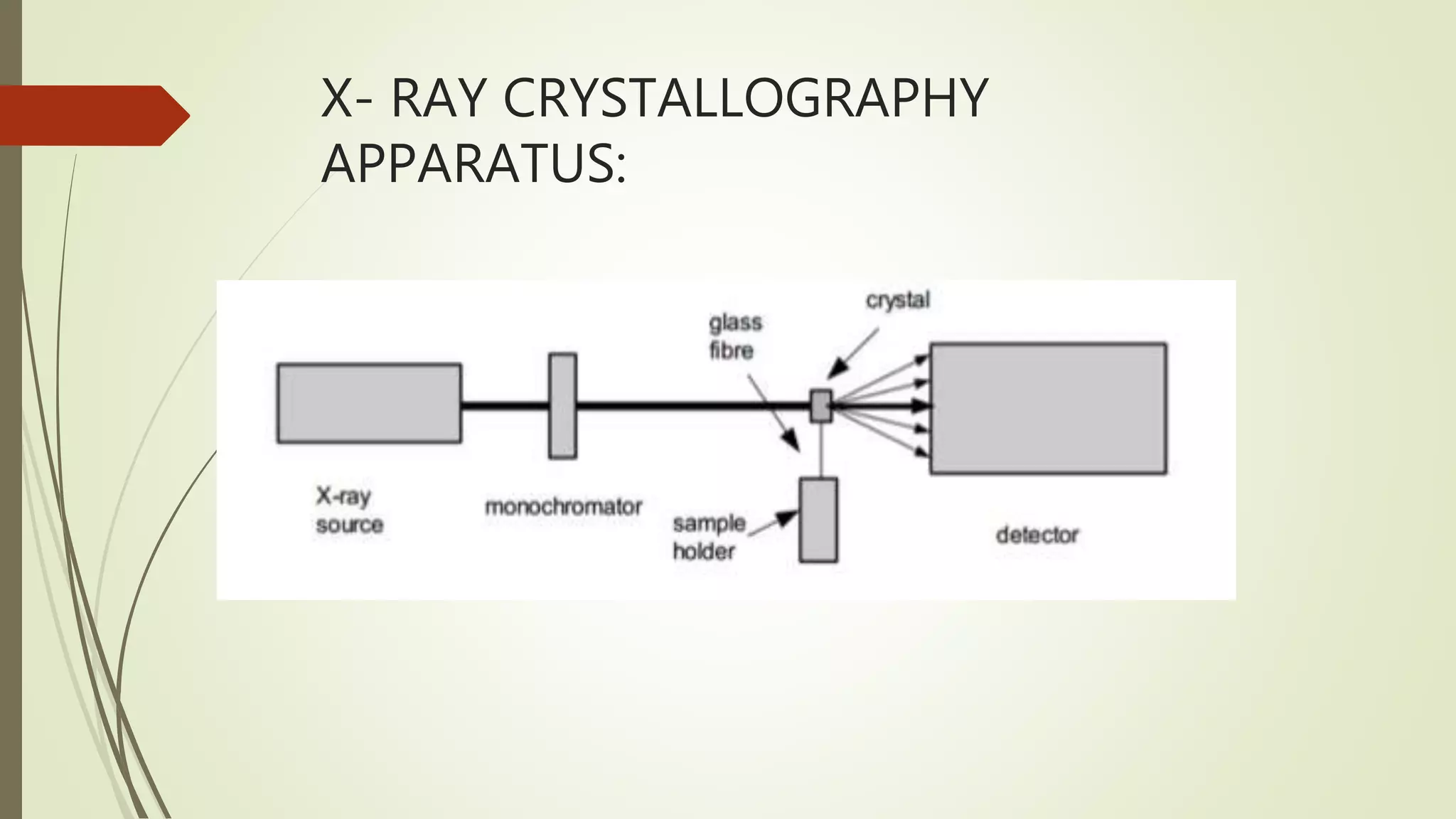
X-ray crystallography is a technique used to determine the atomic structure of crystals. It involves firing X-rays at a crystal and measuring the scattering pattern, which can reveal the positions of atoms within the crystal. Key apparatus include an X-ray source, filters and monochromators to produce a single wavelength beam, a diffractometer to rotate the crystal and detector, and an X-ray detector. The procedure involves growing crystals, collecting diffraction data, solving the crystal structure through methods like molecular replacement, and refining the structural model. X-ray crystallography is used to study biological molecules and materials that form crystals.