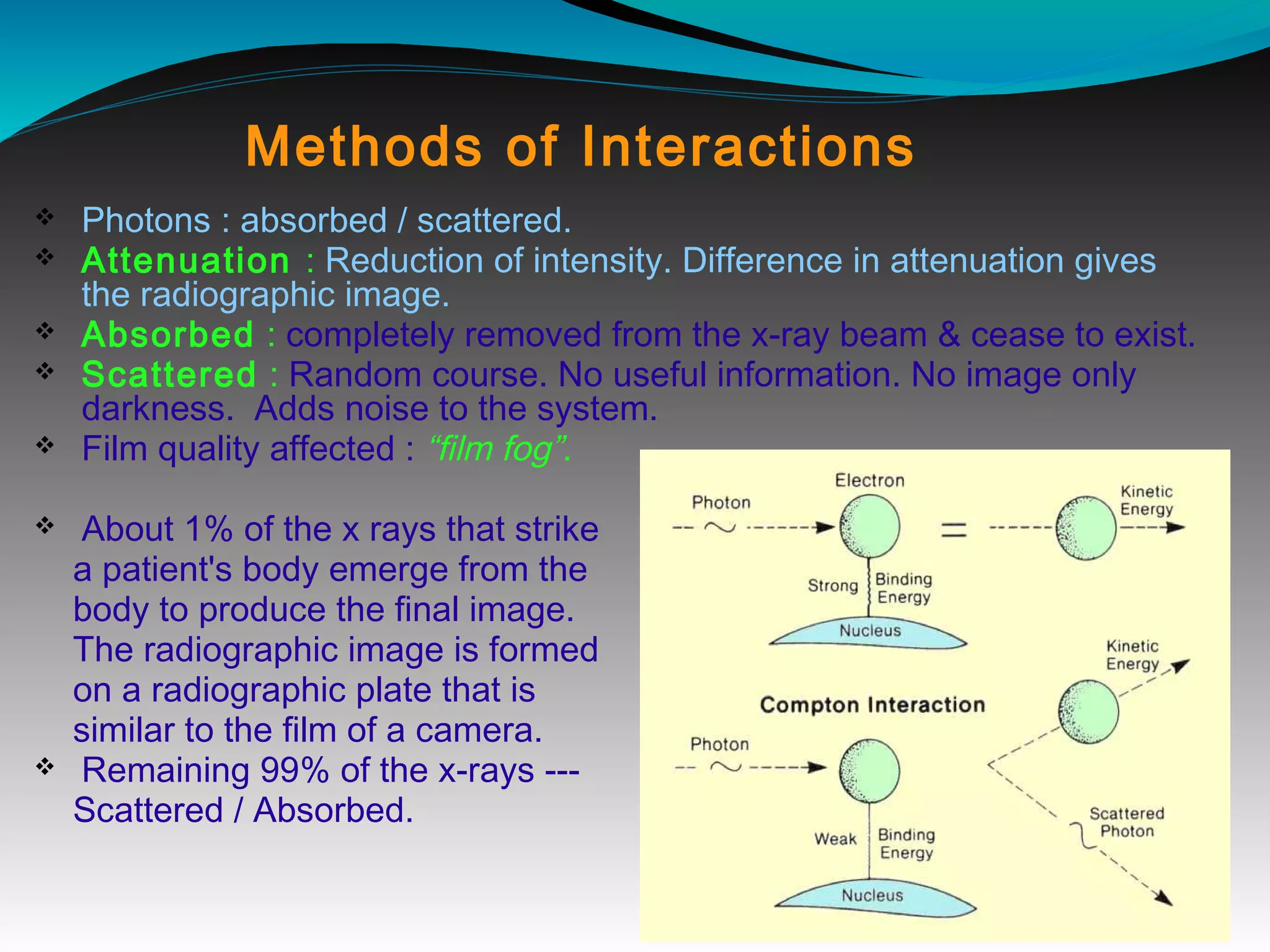
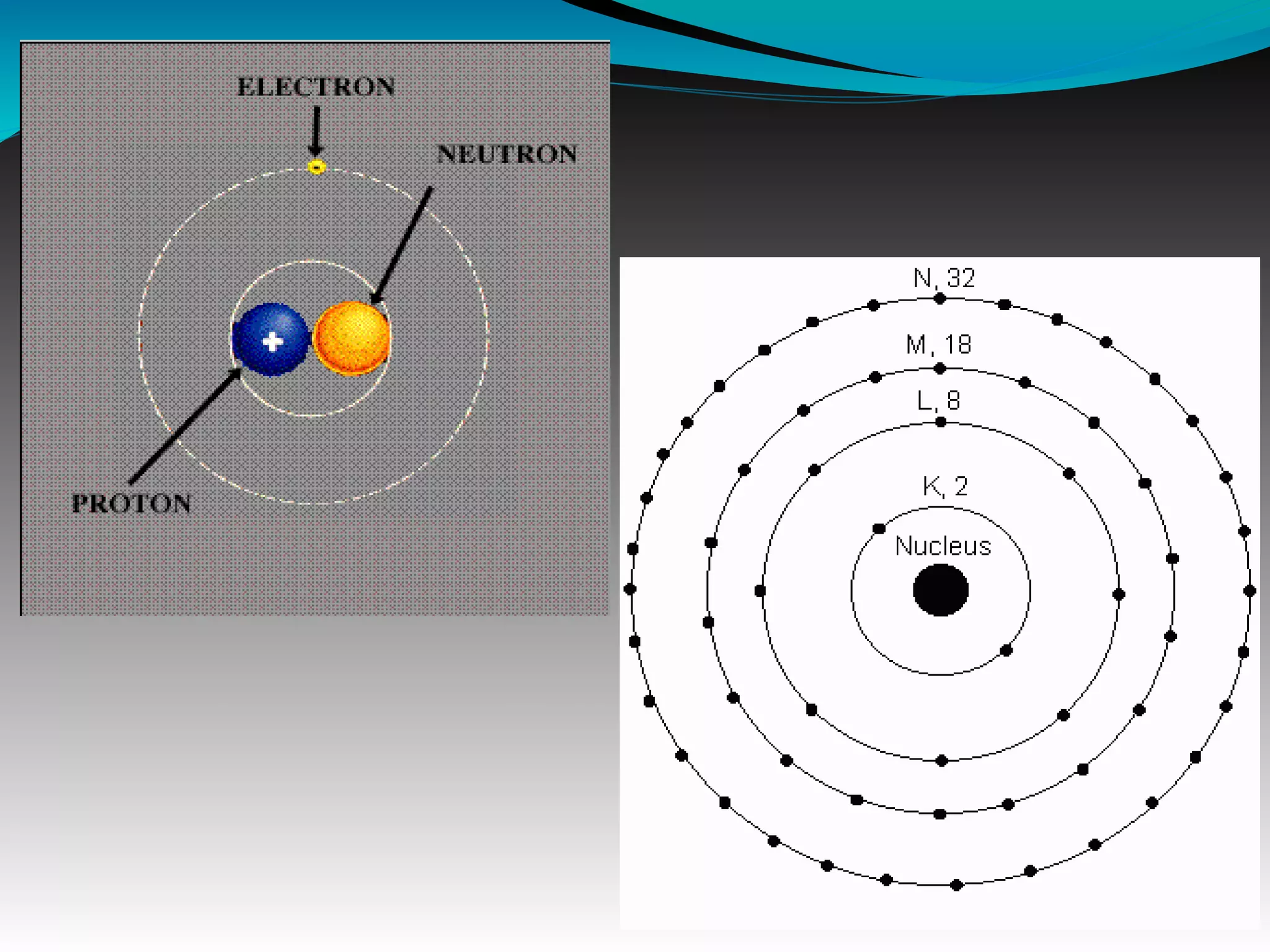
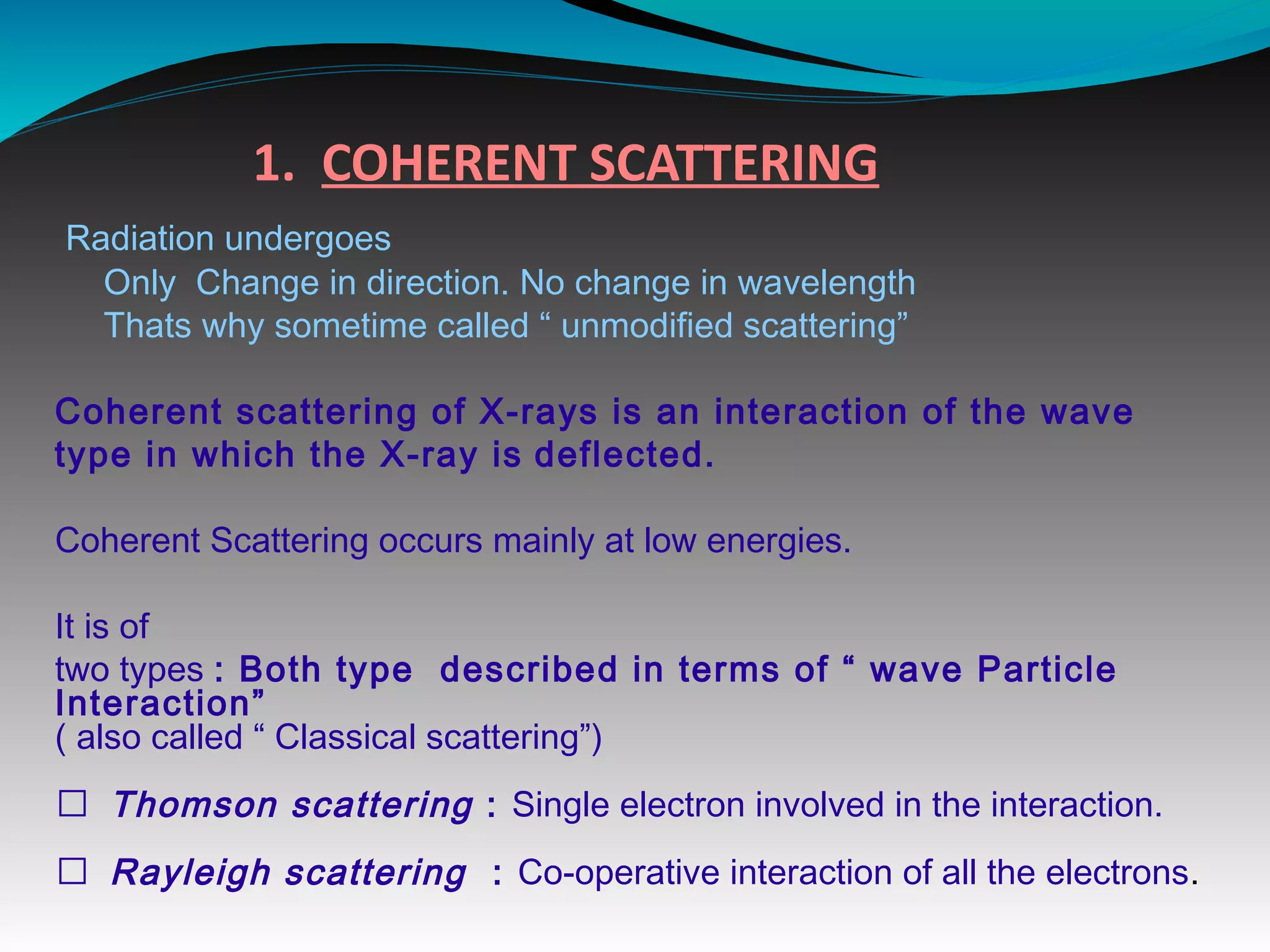
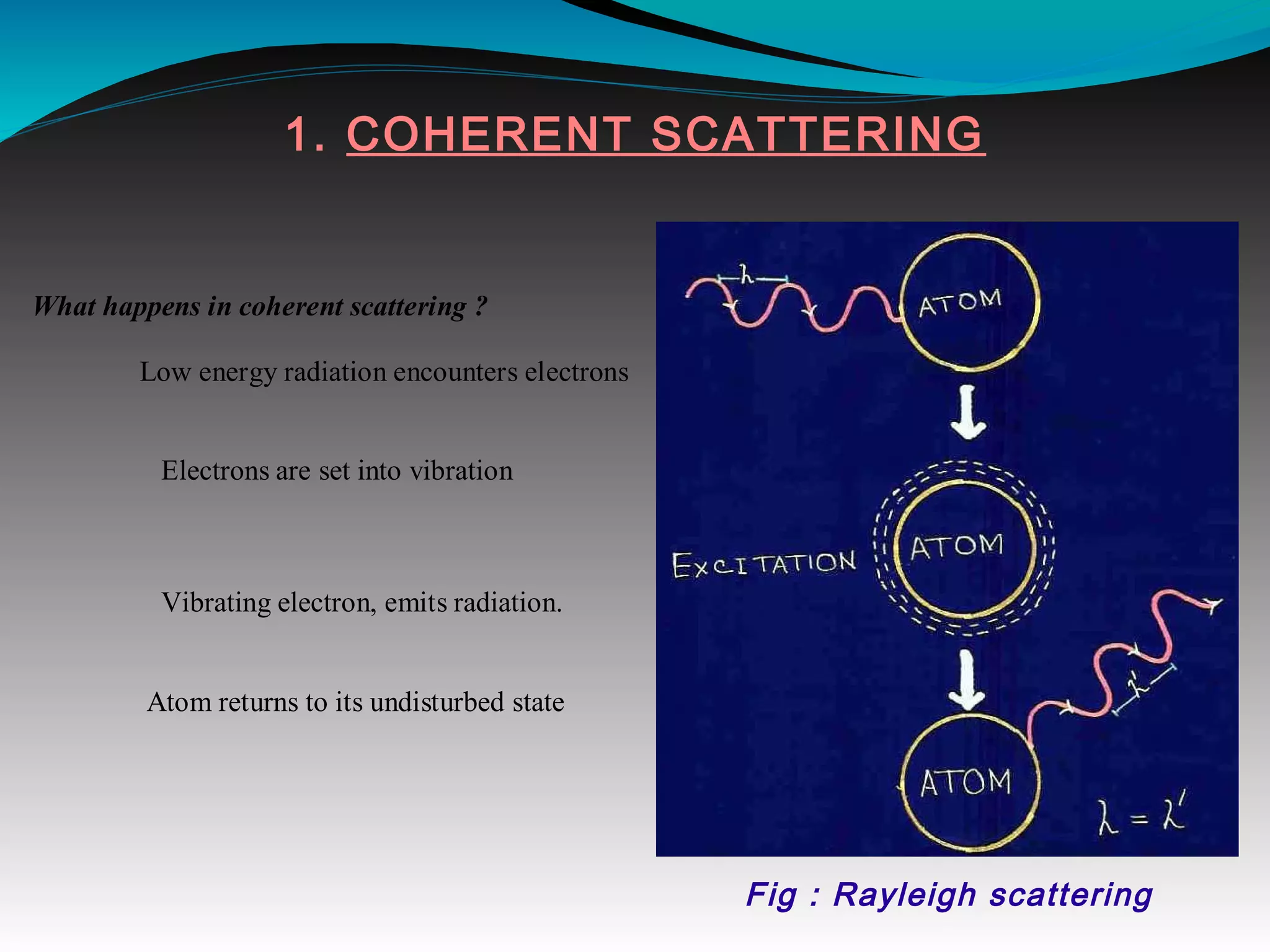
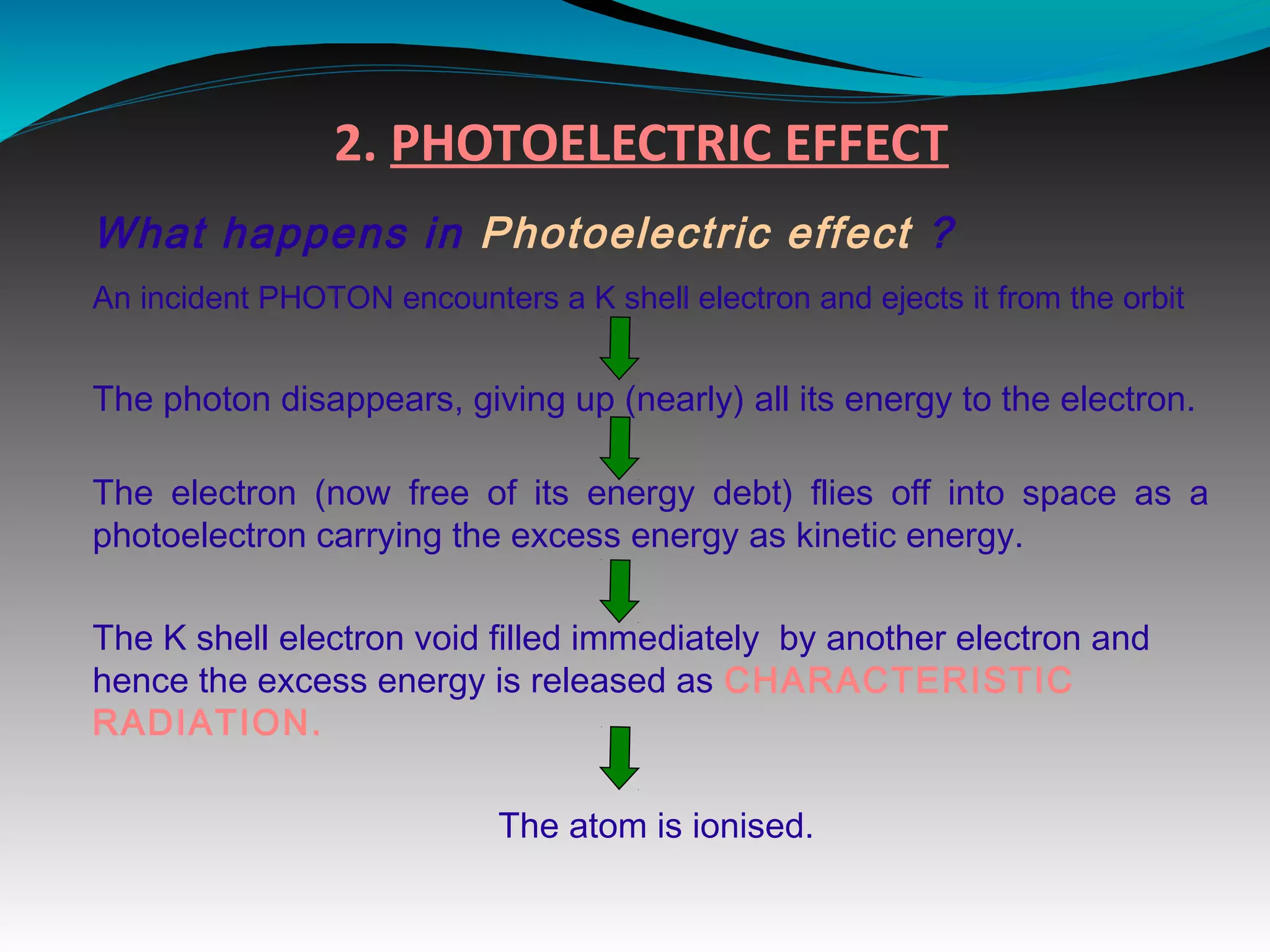
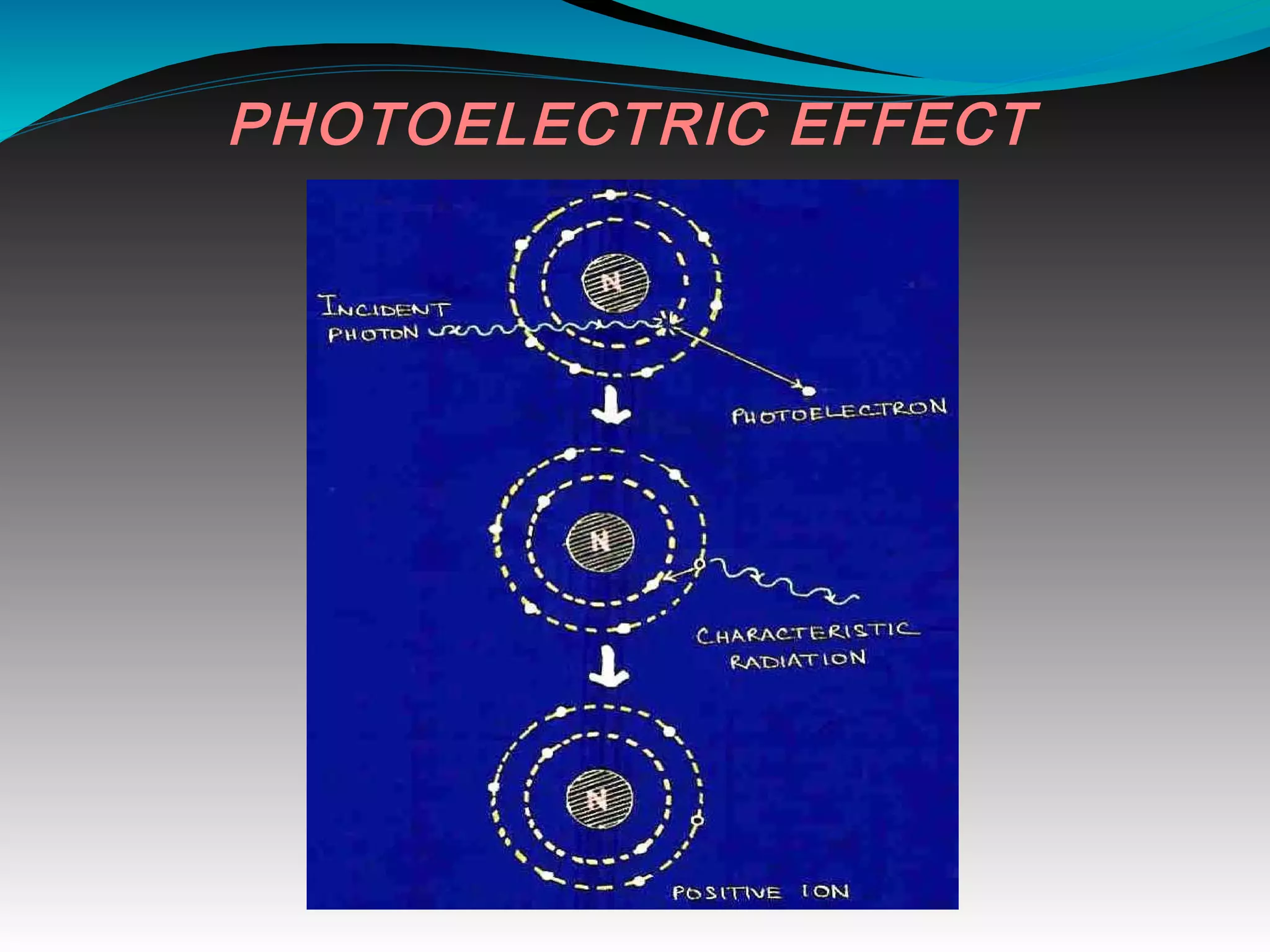
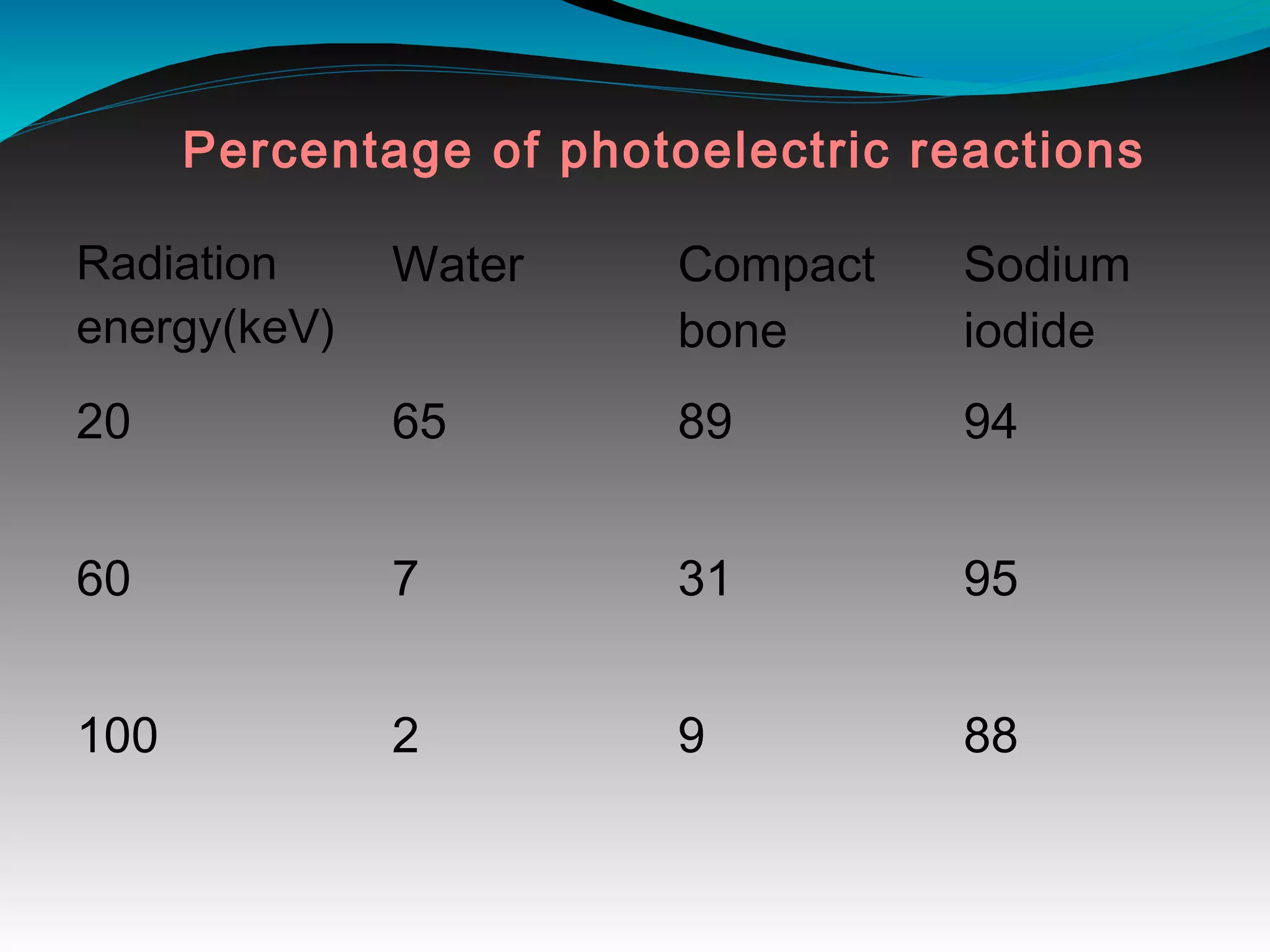
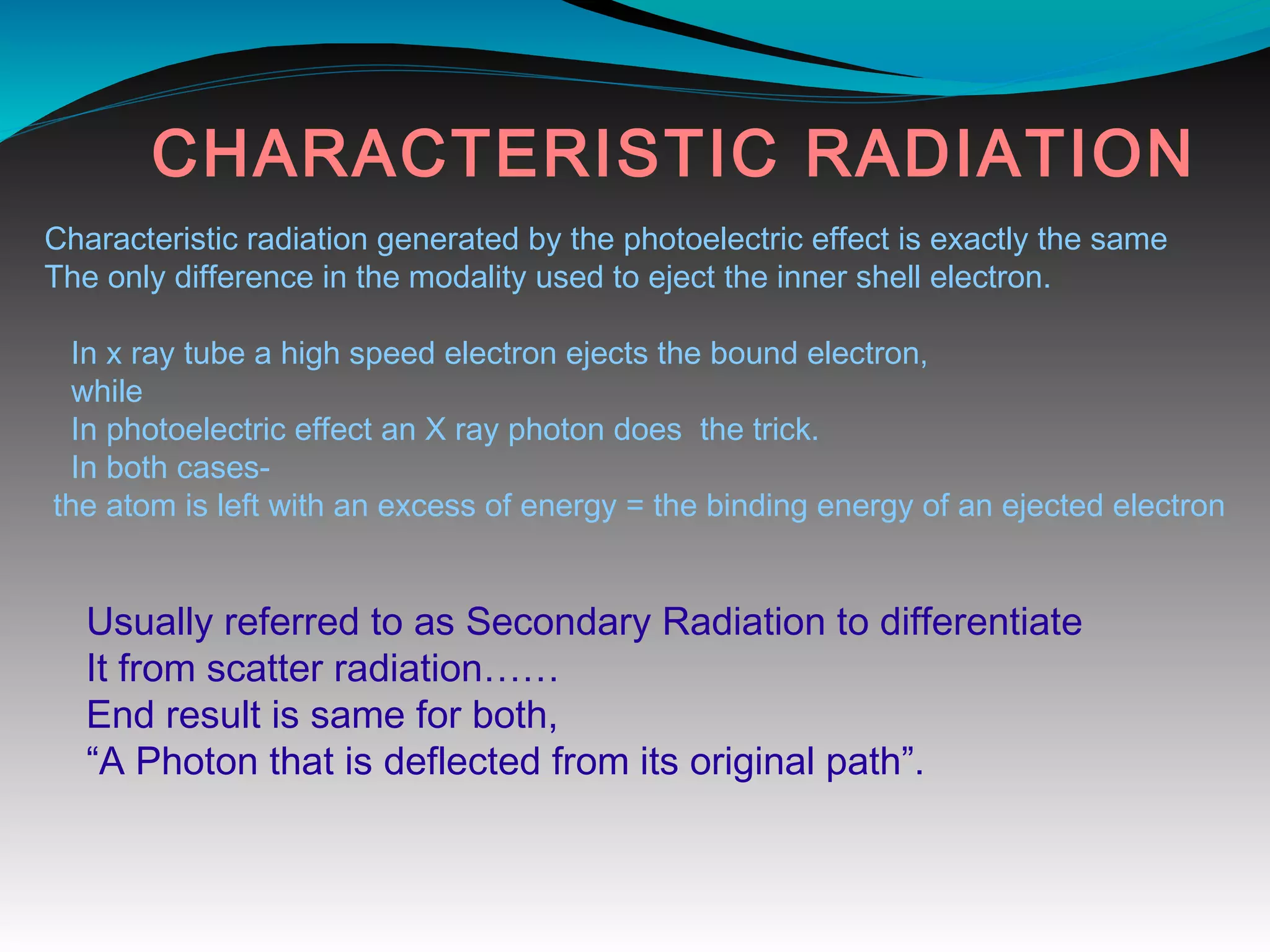
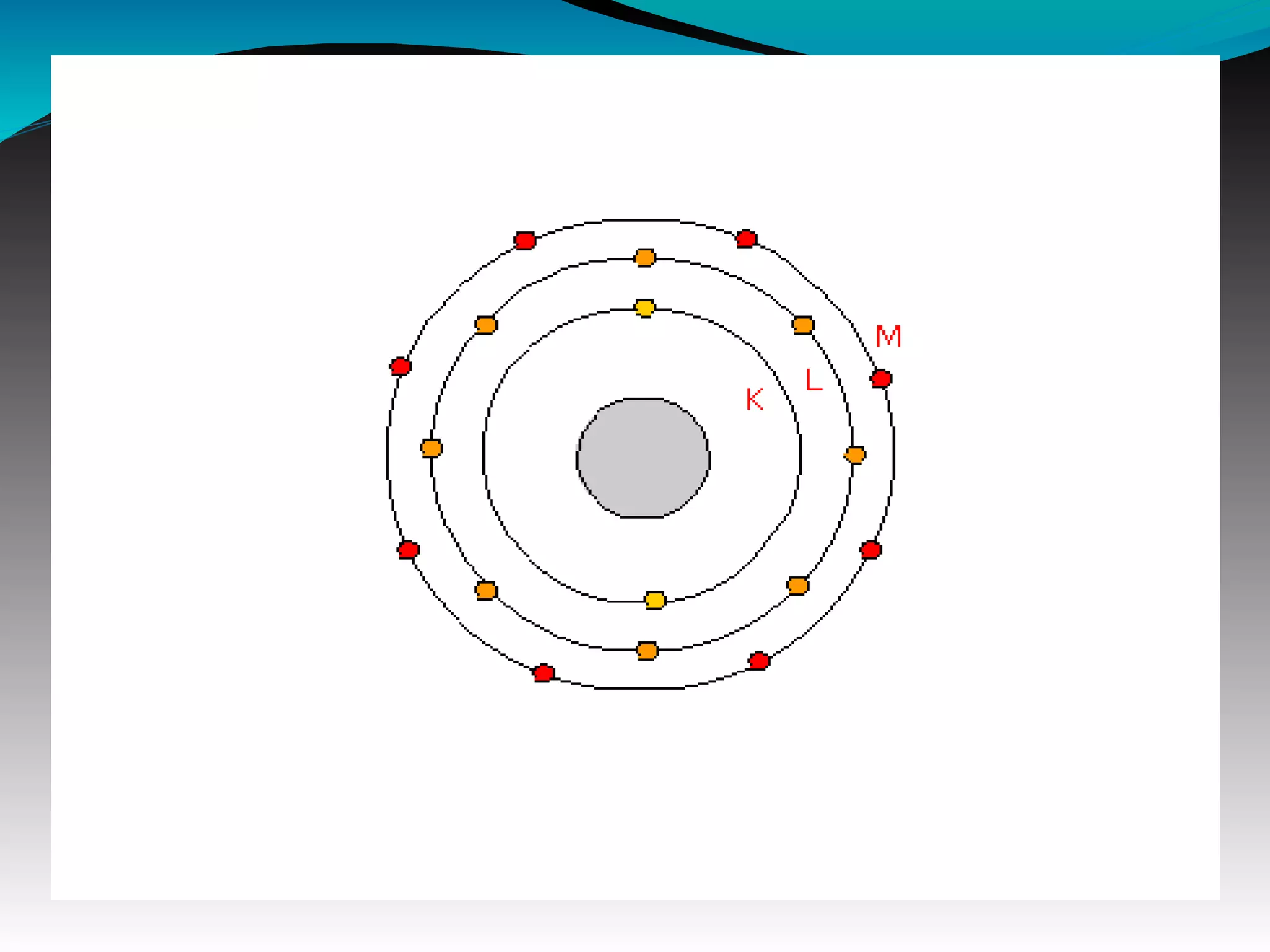
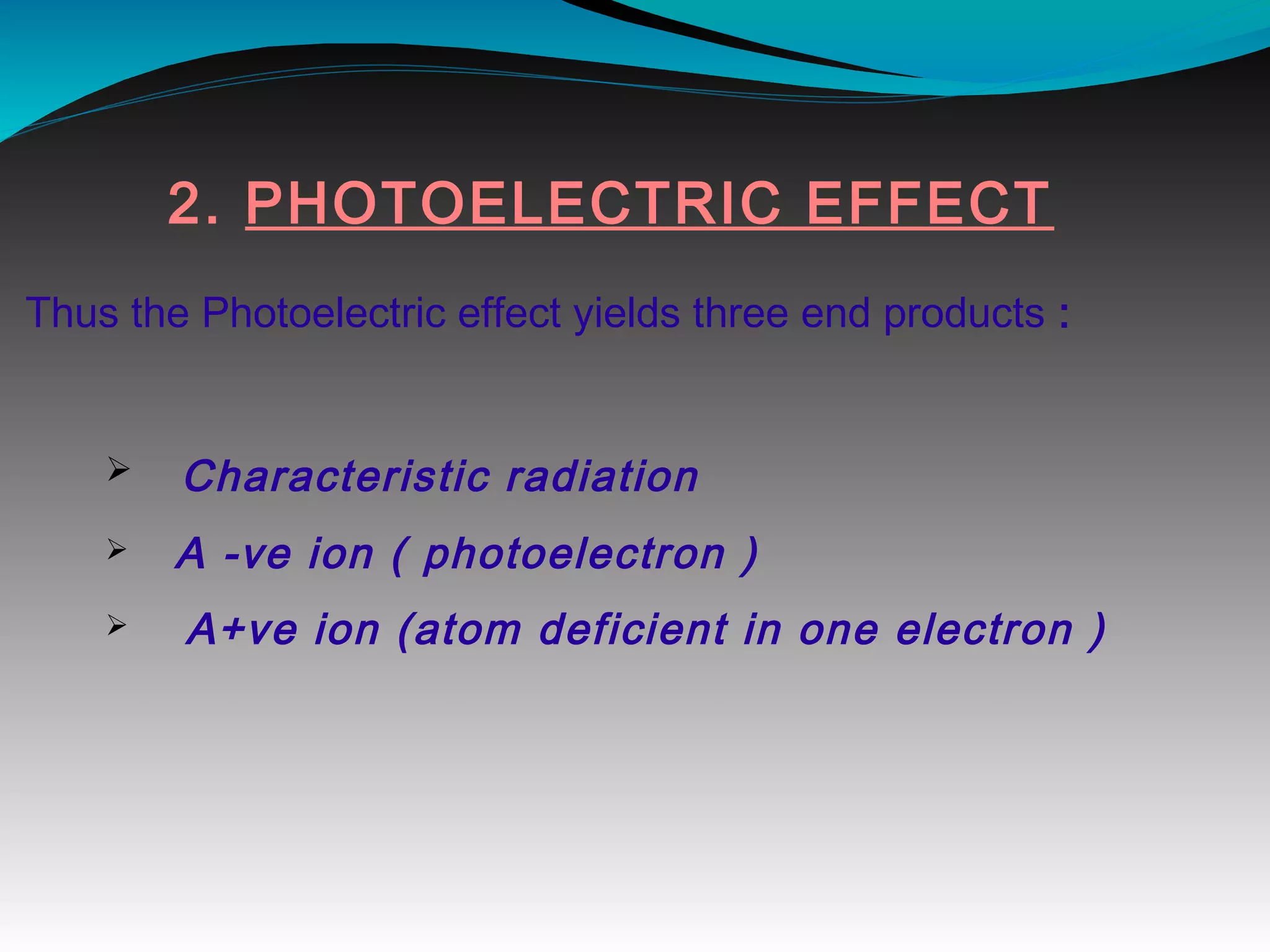
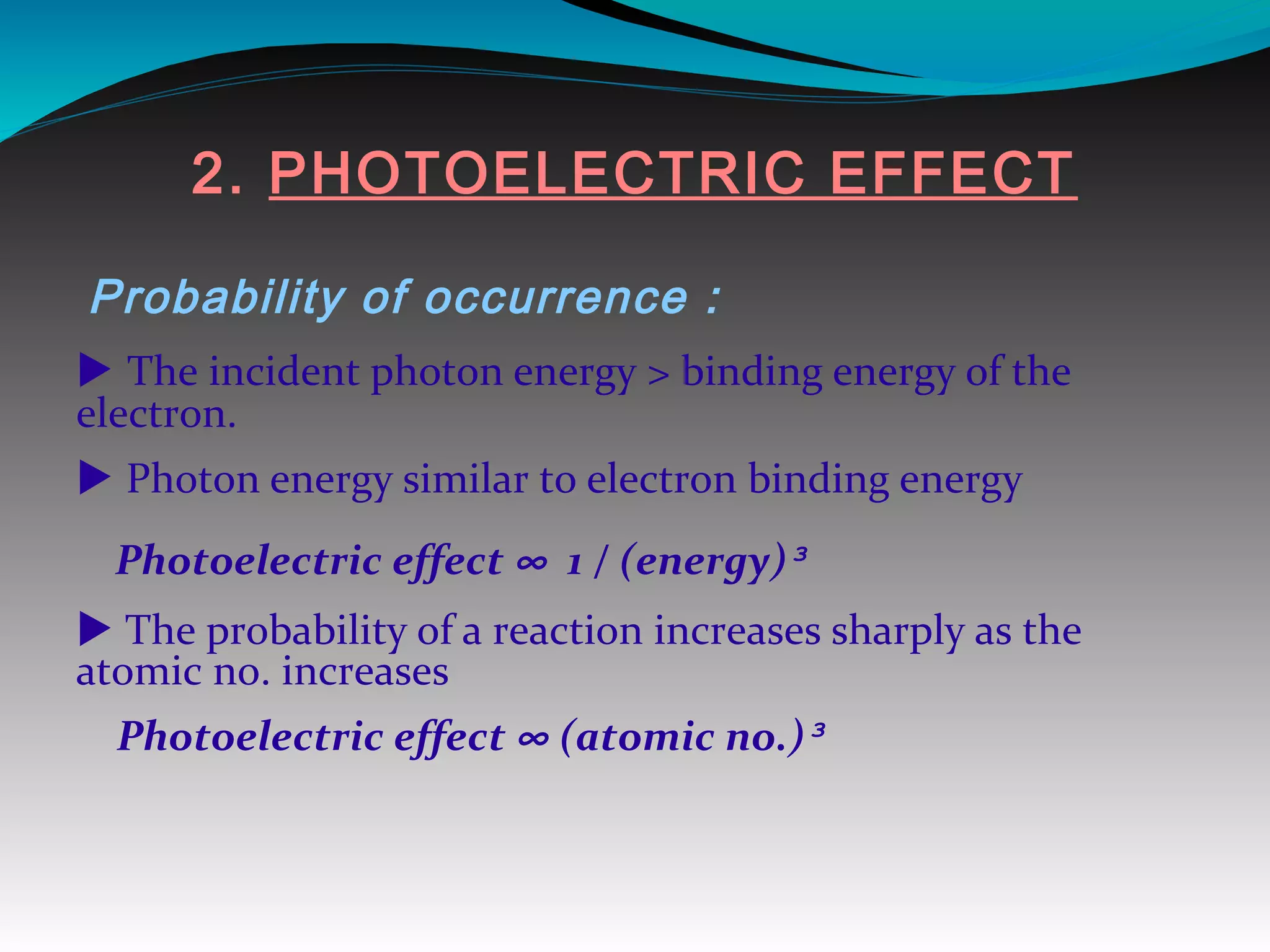
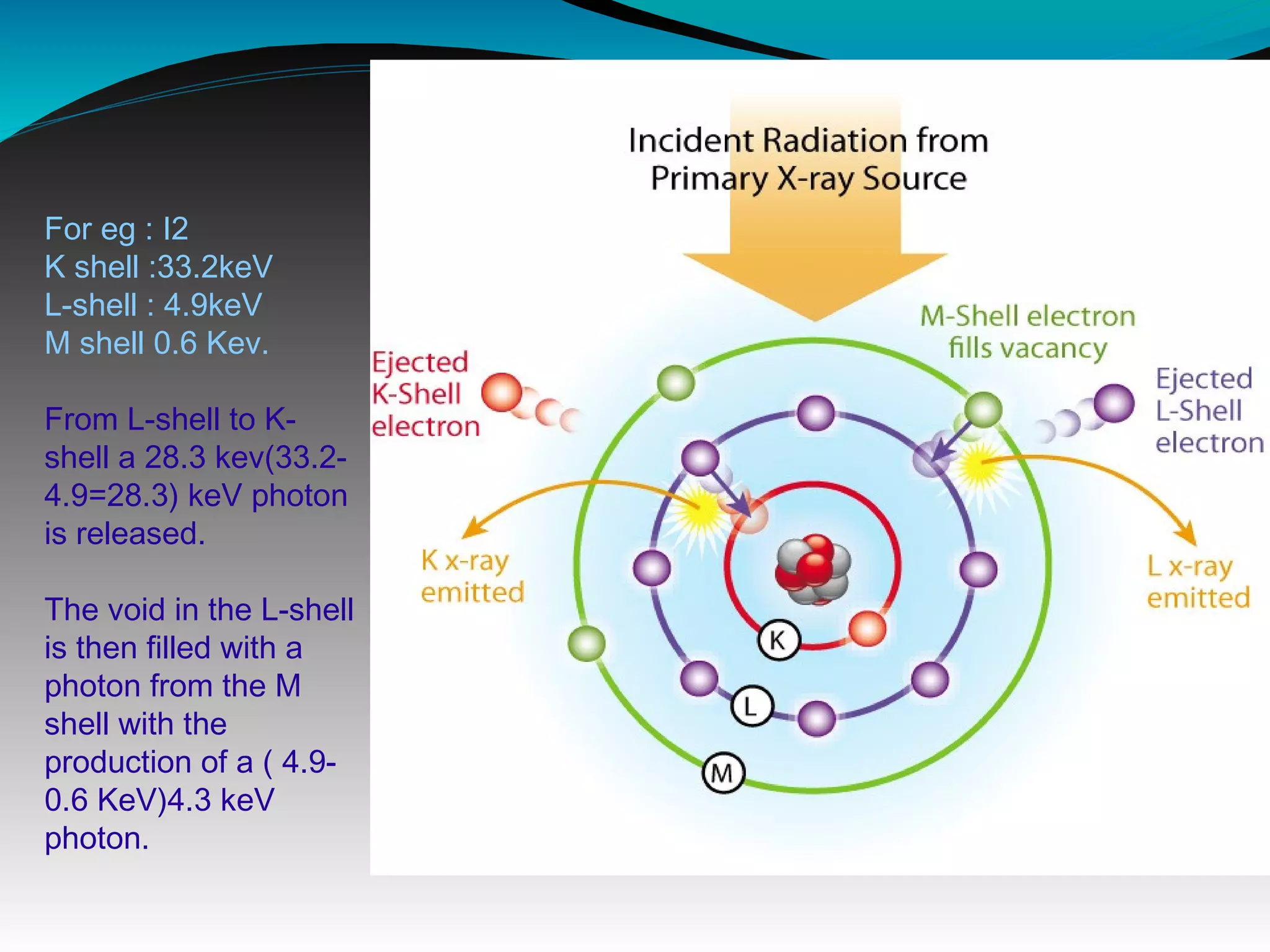
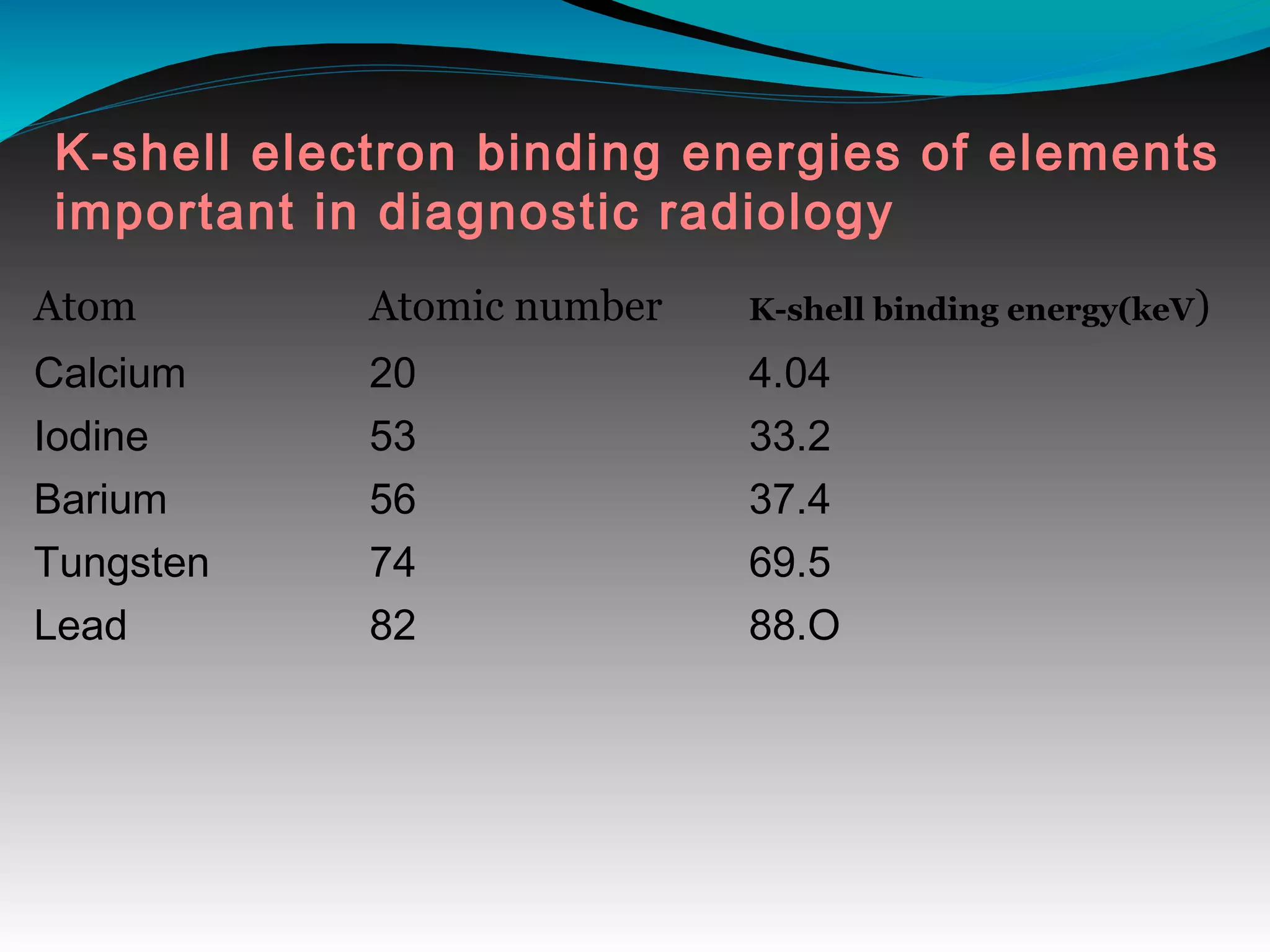
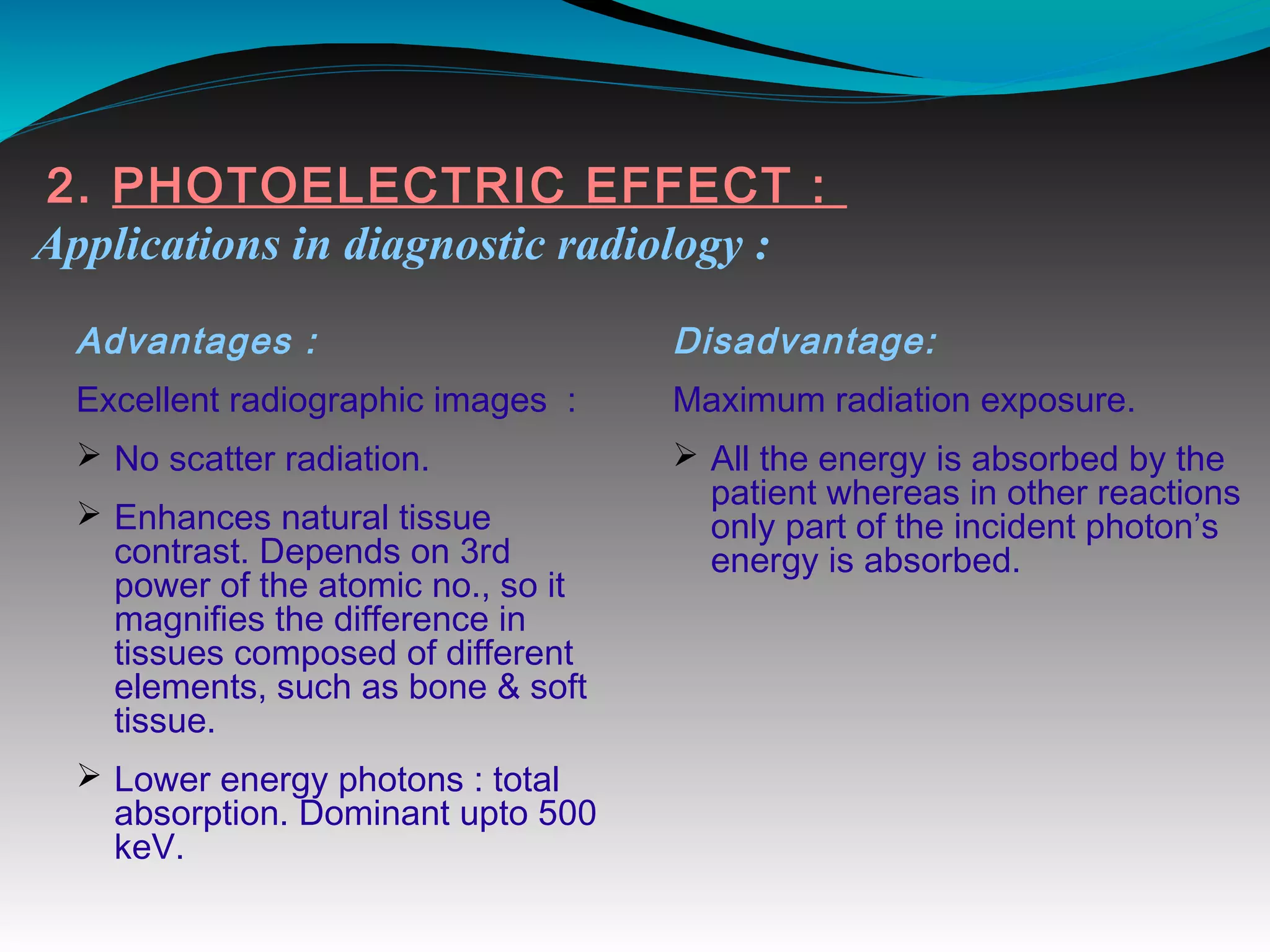
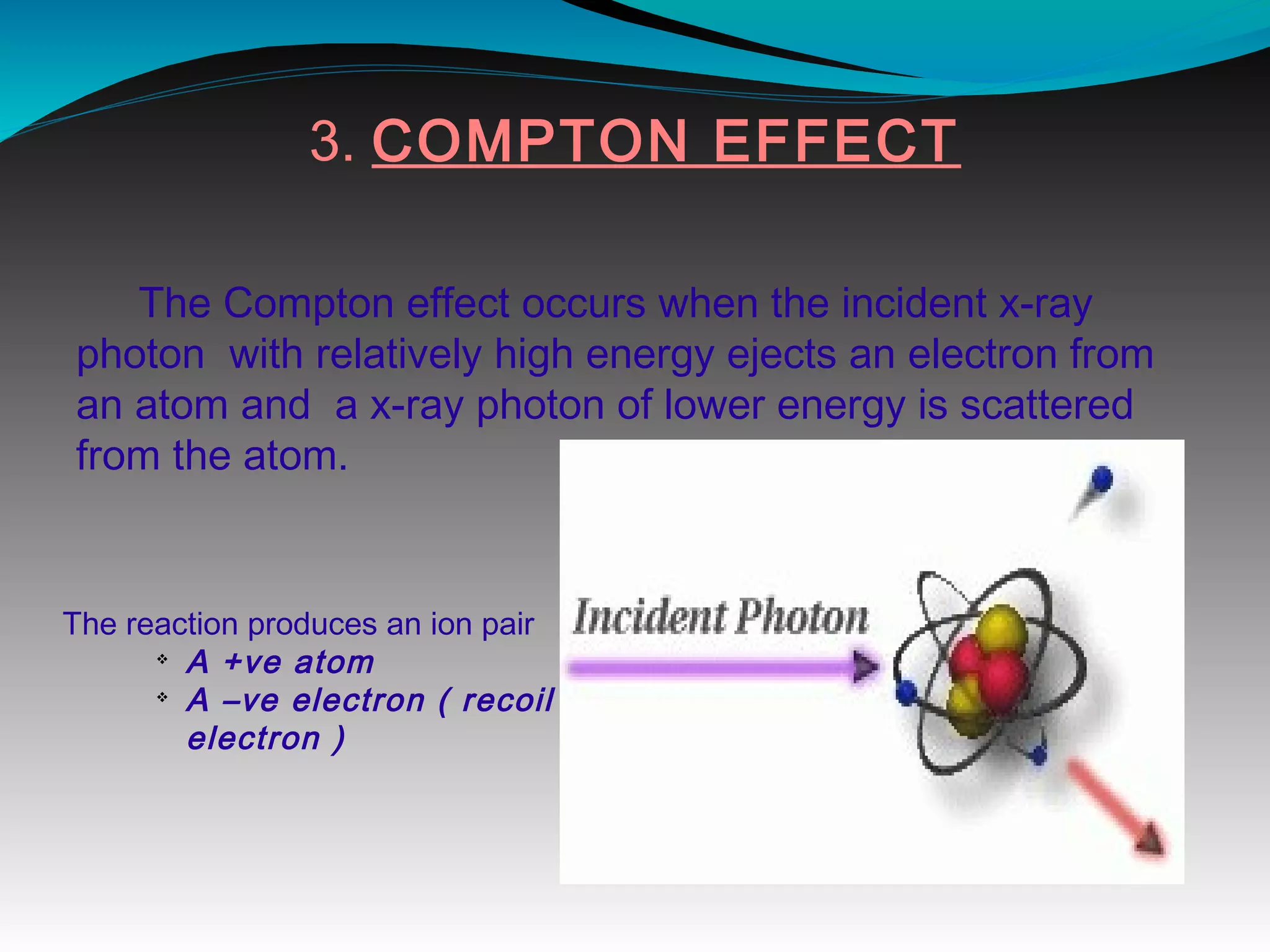
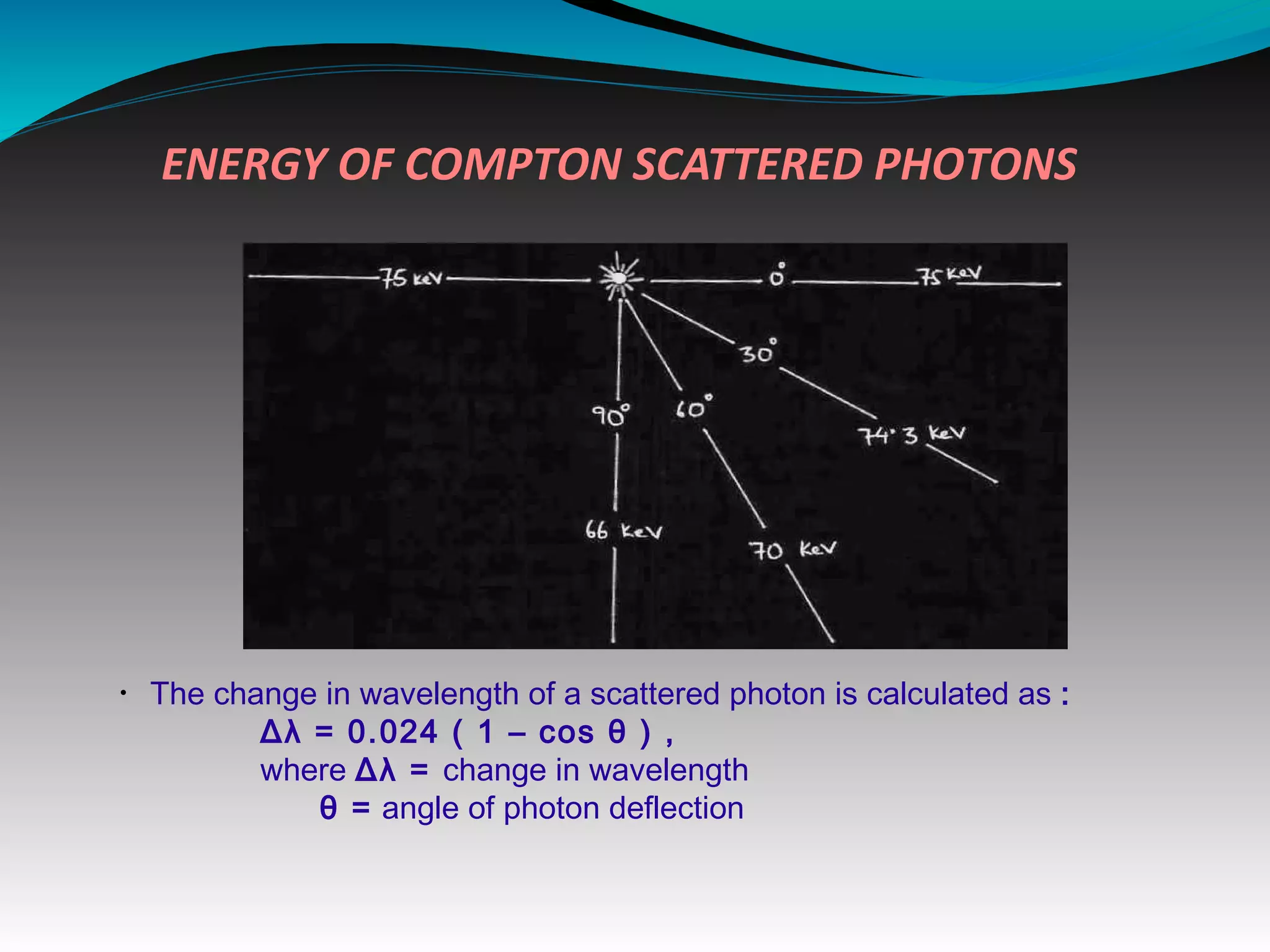
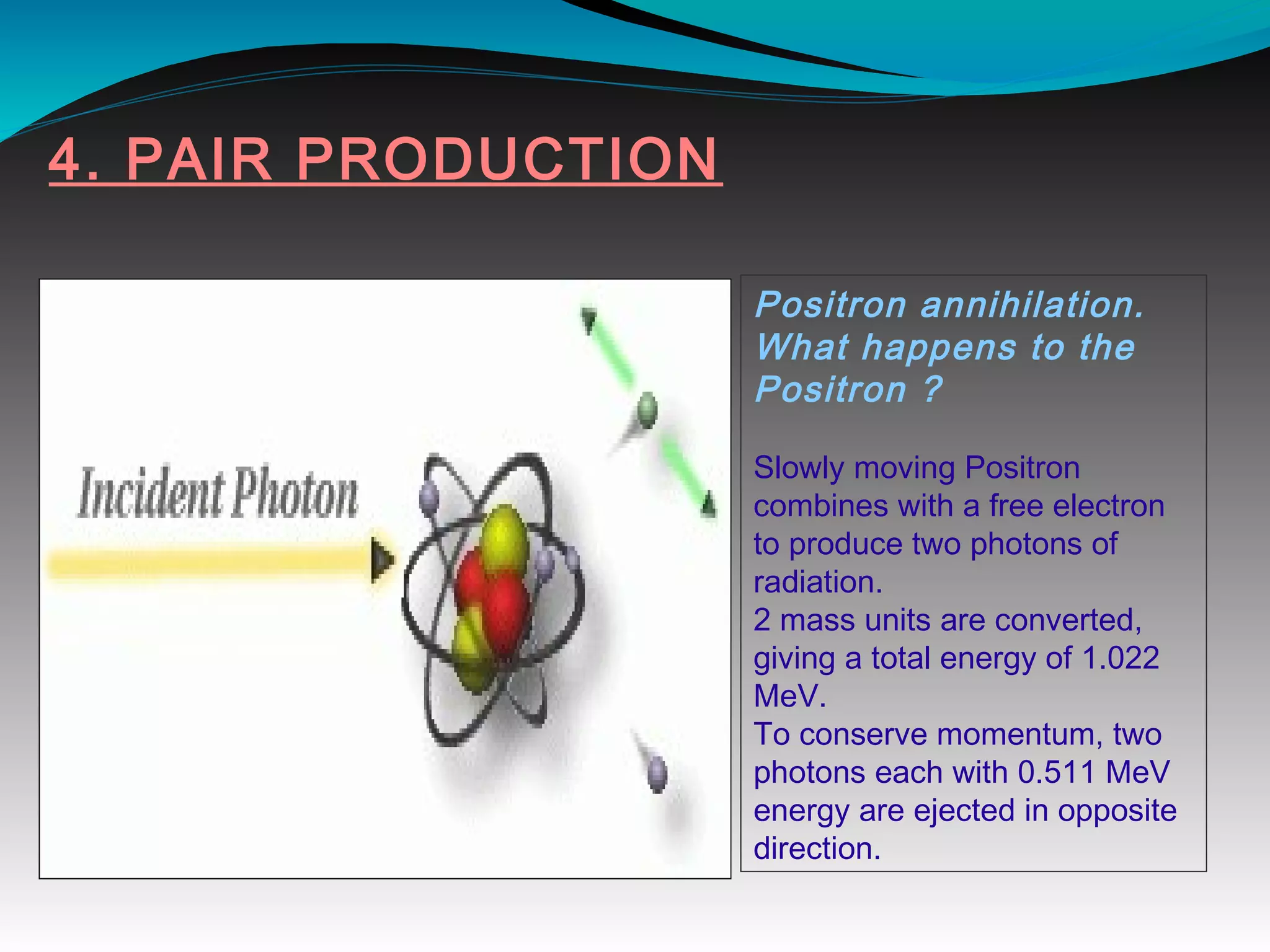
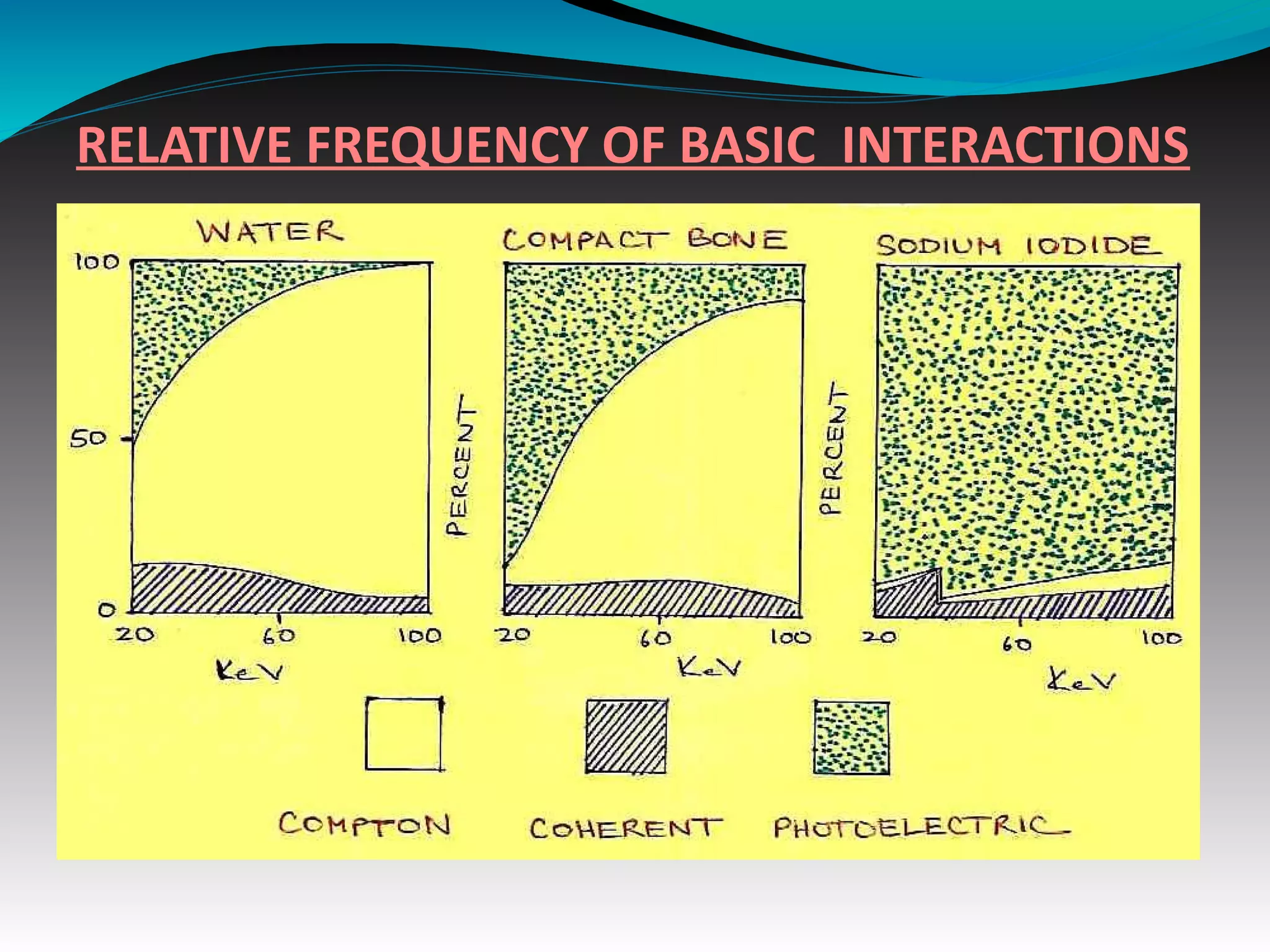
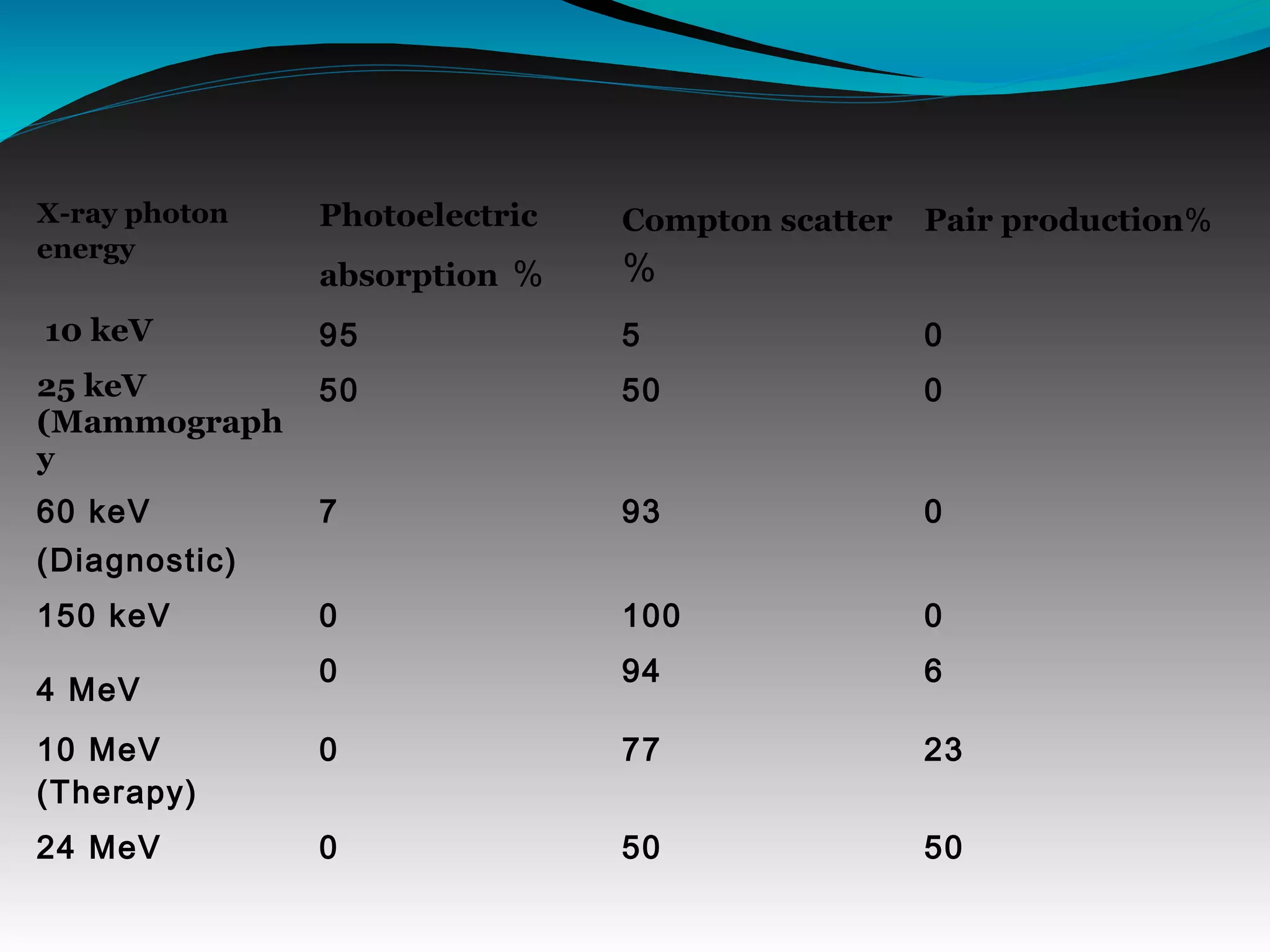
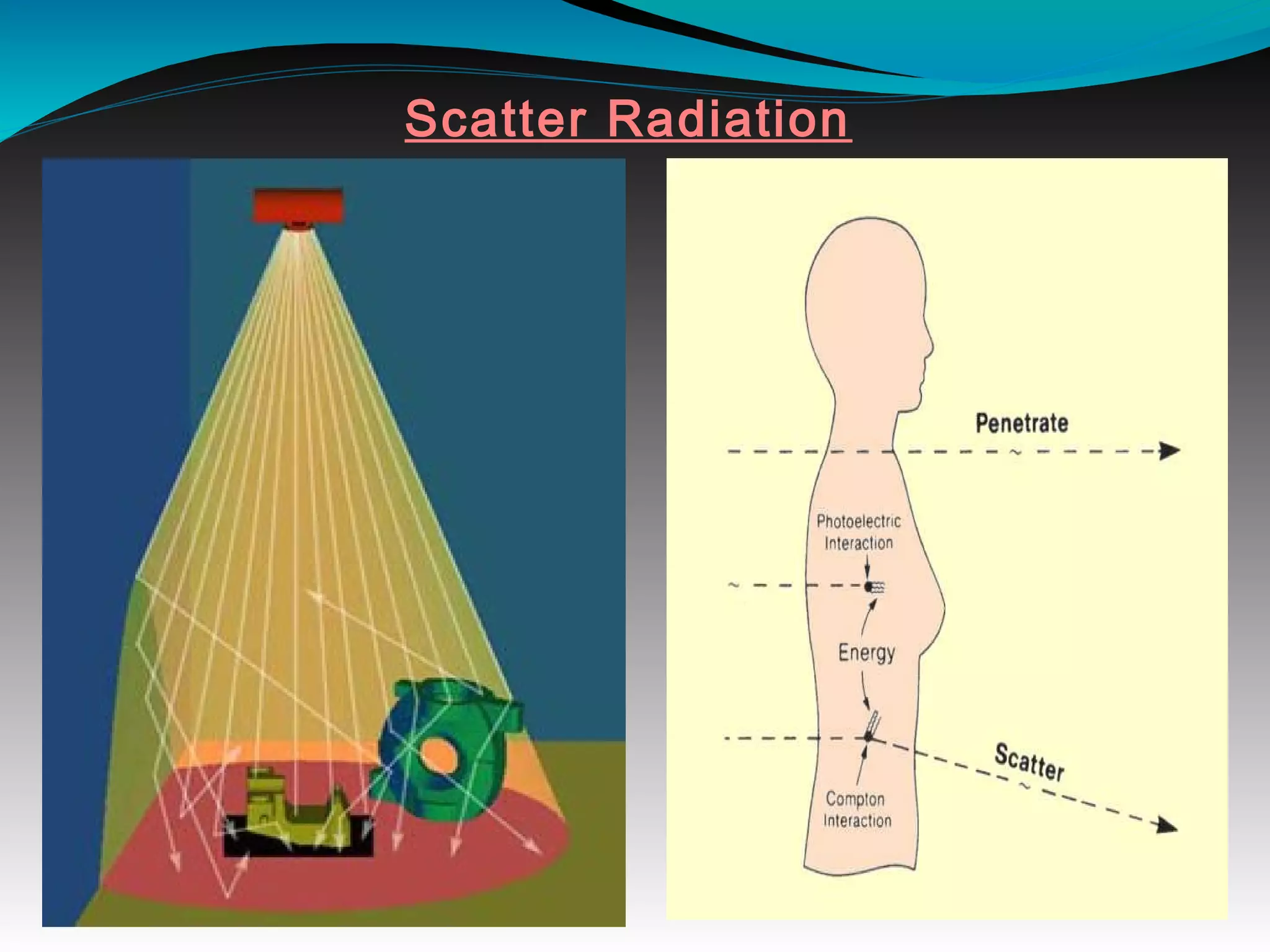
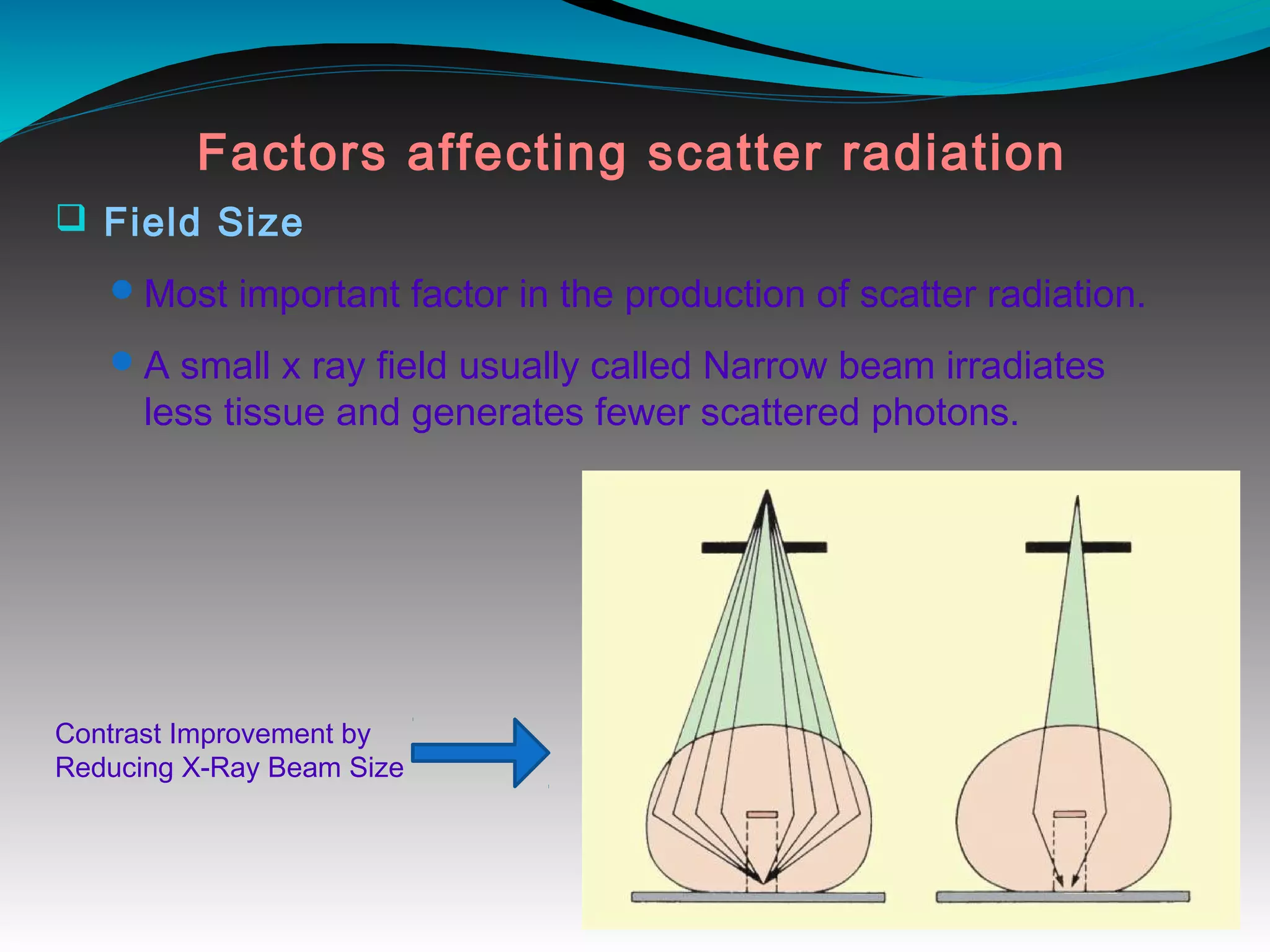
X-rays can interact with matter through various interactions including the photoelectric effect, Compton scattering, and coherent scattering. The photoelectric effect and Compton scattering are the most important interactions in diagnostic radiology. Scatter radiation is a major source of reduced image quality and increased patient dose in x-rays, and various techniques like grids and filters are used to control scatter.