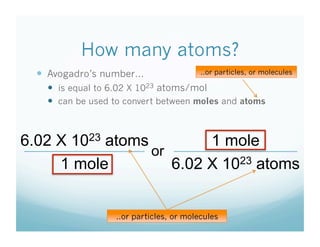
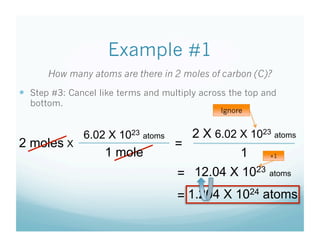
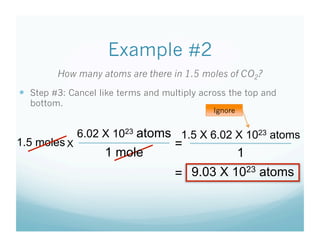
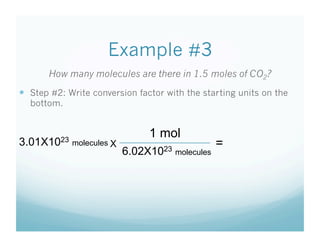
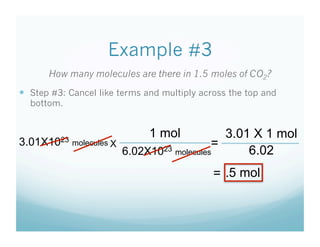
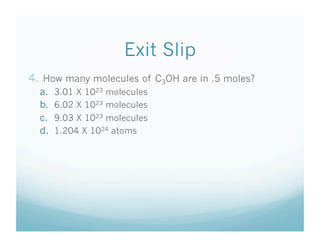
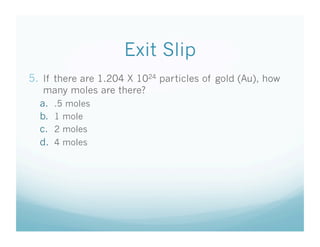
- Students were taught how to convert between moles and atoms/molecules using Avogadro's number as a conversion factor.
- Examples were worked through as a class to show the 3 step process: 1) write starting unit, 2) write conversion factor with starting units on bottom, 3) cancel units and multiply.
- Students then worked in pairs on whiteboard practice questions applying the method.
- An exit slip quiz assessed students' understanding of key concepts like Avogadro's number and performing mole-to-particle conversions.
- Homework assigned was to finish the practice question worksheet.