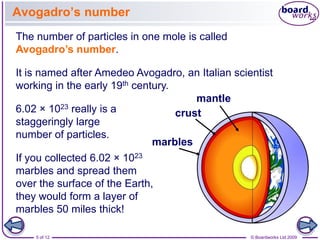
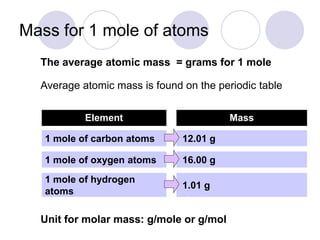
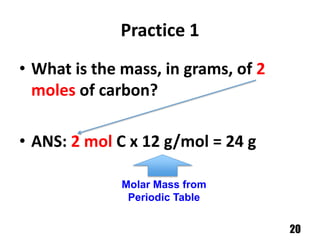
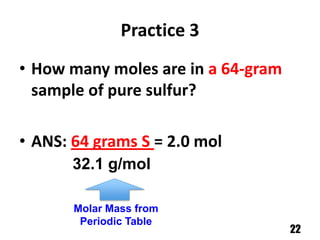
A counting unit called a mole is used in chemistry to quantify large numbers of small particles like atoms and molecules. A mole is defined as 6.02 x 1023 particles and can be used to relate the mass of a substance in grams to the number of particles or formula units present. For example, 12 grams of carbon contains 1 mole or 6.02 x 1023 carbon atoms, and 18 grams of water contains 1 mole or 6.02 x 1023 water molecules. Converting between moles and mass uses the molar mass of an element found on the periodic table.