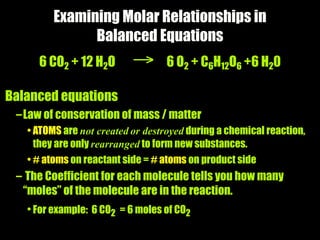
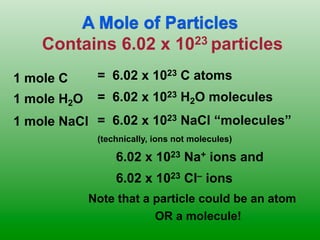
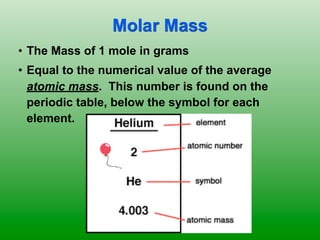
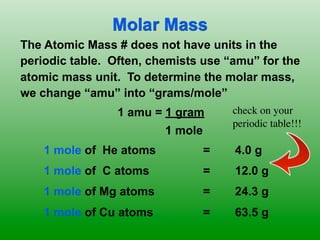
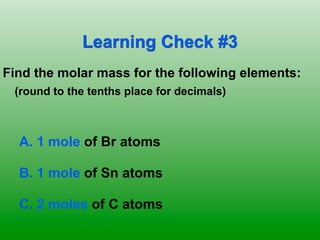
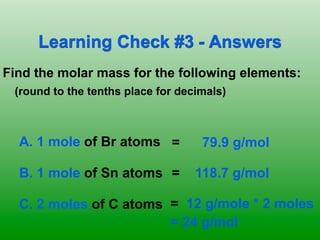
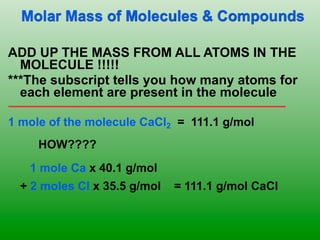
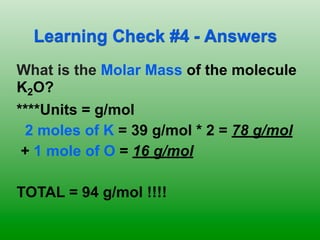
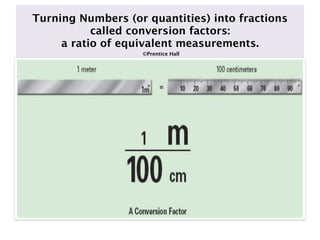
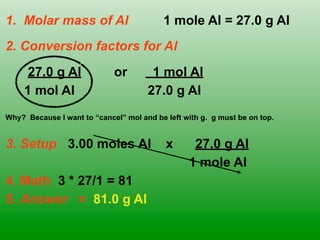
The molar mass of aluminum is 27.0 g/mol. To convert 3.00 moles of aluminum to grams, a conversion factor of 27.0 g Al/1 mol Al is used. Multiplying 3.00 mol Al by the conversion factor gives 81.0 g Al. Dimensional analysis and conversion factors allow quantities in moles and grams to be interconverted for aluminum using its molar mass.