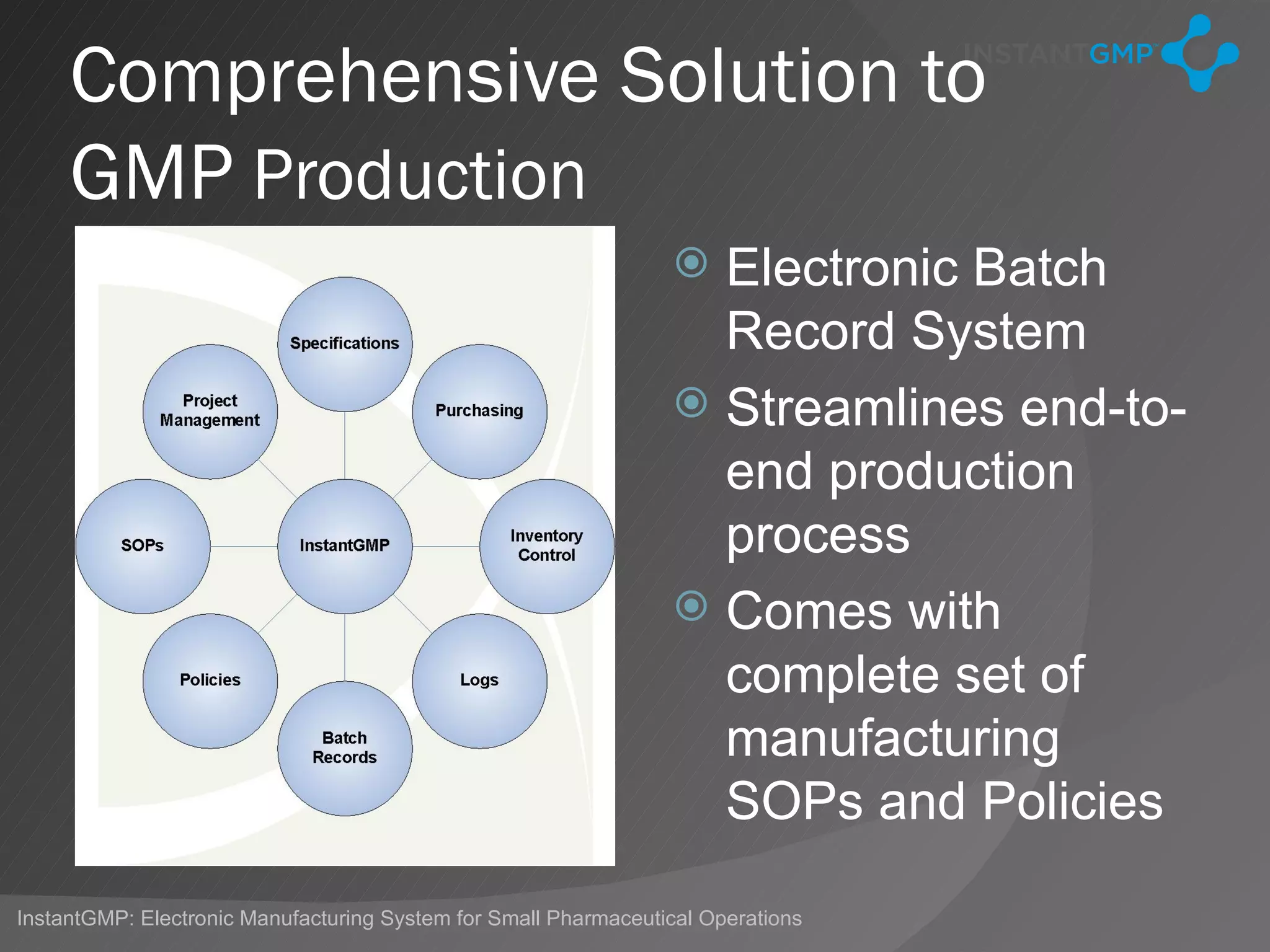
The document discusses Good Manufacturing Practices (GMP) in India, highlighting the differences in compliance levels across countries and the specific challenges faced by Indian pharmaceutical companies. It points out that while Indian firms are not required to follow complex global certification processes, they still must adhere to certain FDA inspections for U.S.-bound products. The InstantGMP system aims to simplify compliance through an electronic batch record system, although significant hurdles related to cost and regulatory adherence remain for many Indian manufacturers.