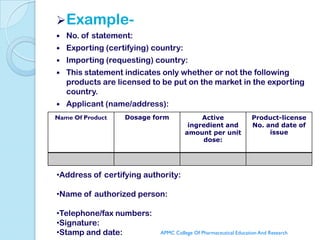
The document discusses the WHO certification scheme for pharmaceutical products. It provides details on the history and revisions of the WHO certification scheme. It describes the types of certificates that can be issued under the scheme including certificates of pharmaceutical products, statements of licensing status, and batch certificates. It outlines the guidelines for participation in the scheme, requesting and issuing certificates, and procedures for investigating quality defects. The aim of the scheme is to help assure good manufacturing practices and quality of pharmaceutical products being exported between member states.