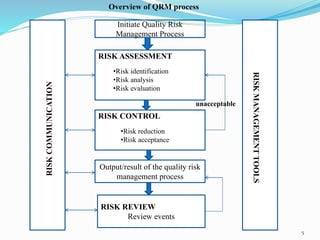
This document discusses quality risk management (QRM) in the pharmaceutical industry. It begins by introducing QRM and its importance in ensuring quality systems. The document then outlines the scope of QRM, including its application across various stages of drug development and manufacturing. The core principles and process of QRM are described, including risk assessment, control, communication, and review. Various risk management tools are also introduced. Finally, the document discusses integrating QRM into industry and regulatory operations to facilitate consistent decision making.