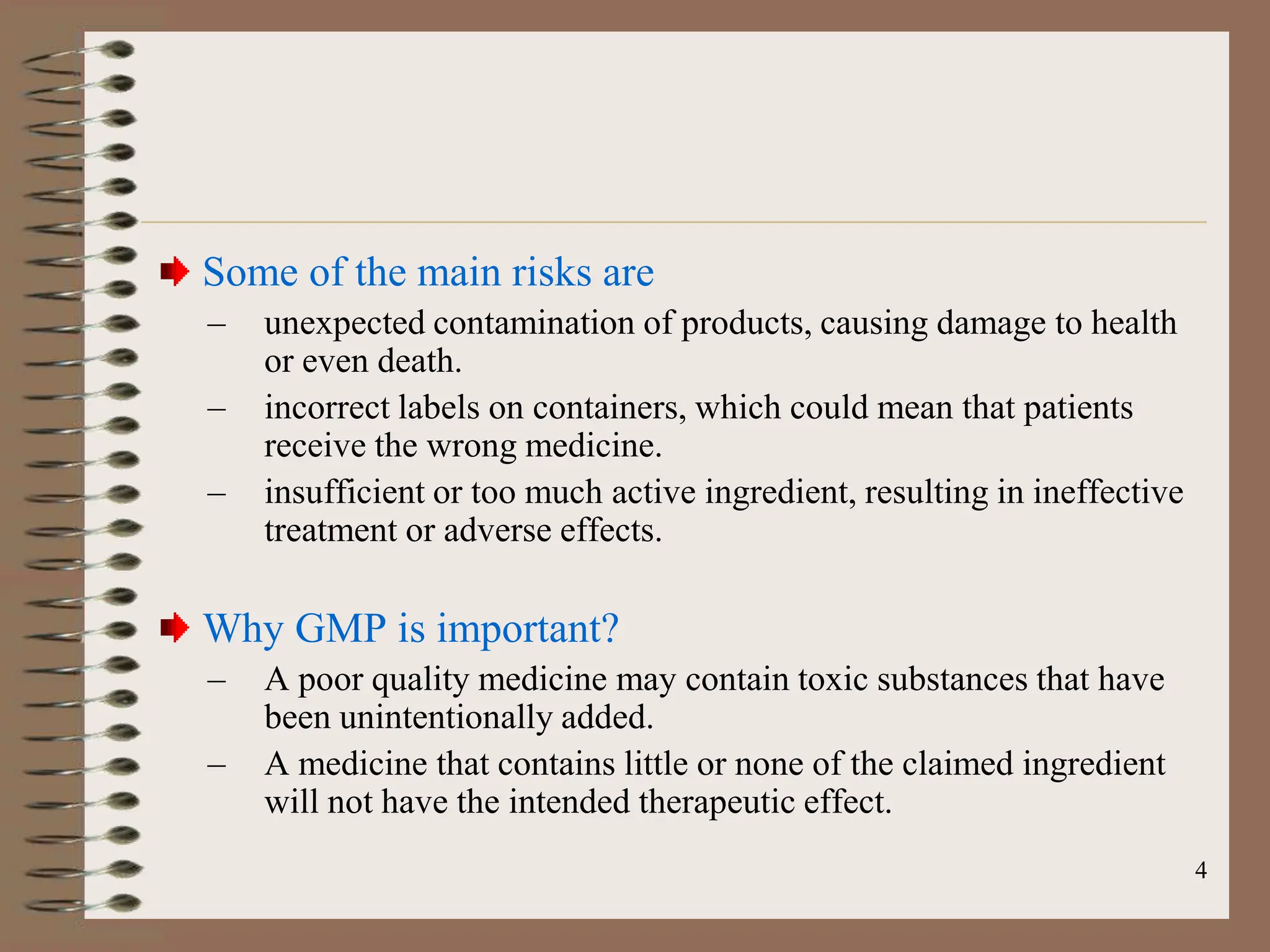
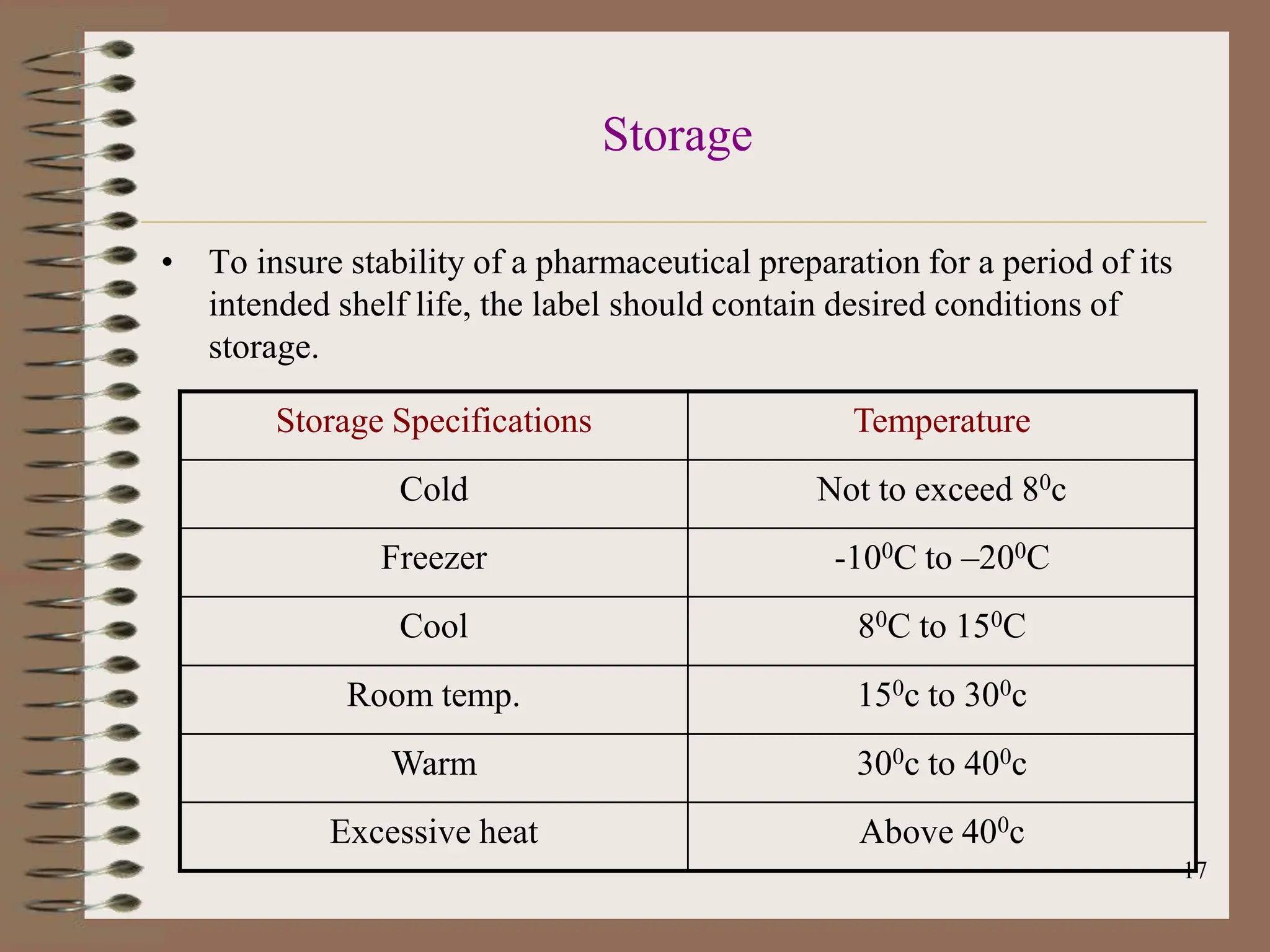
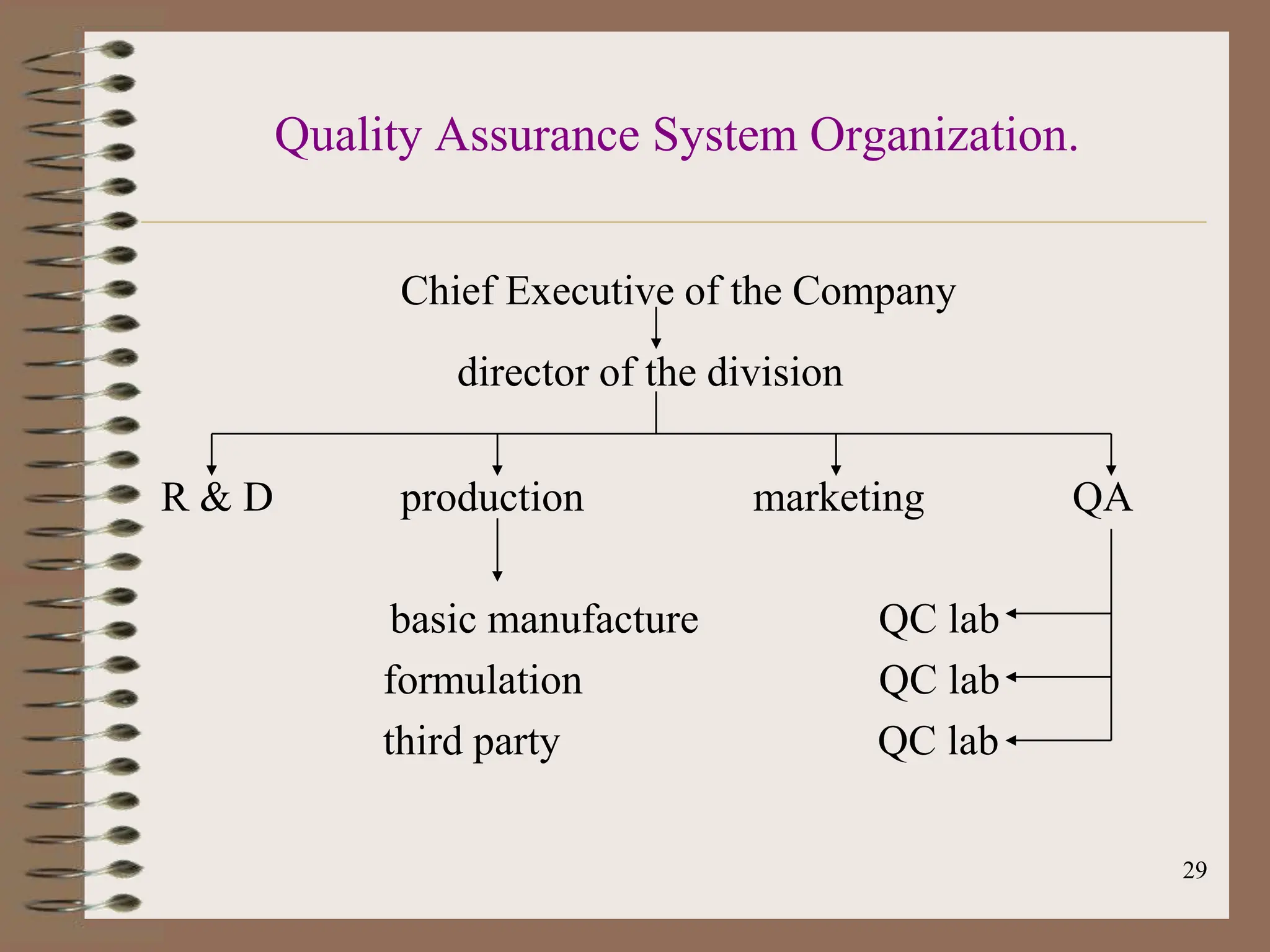
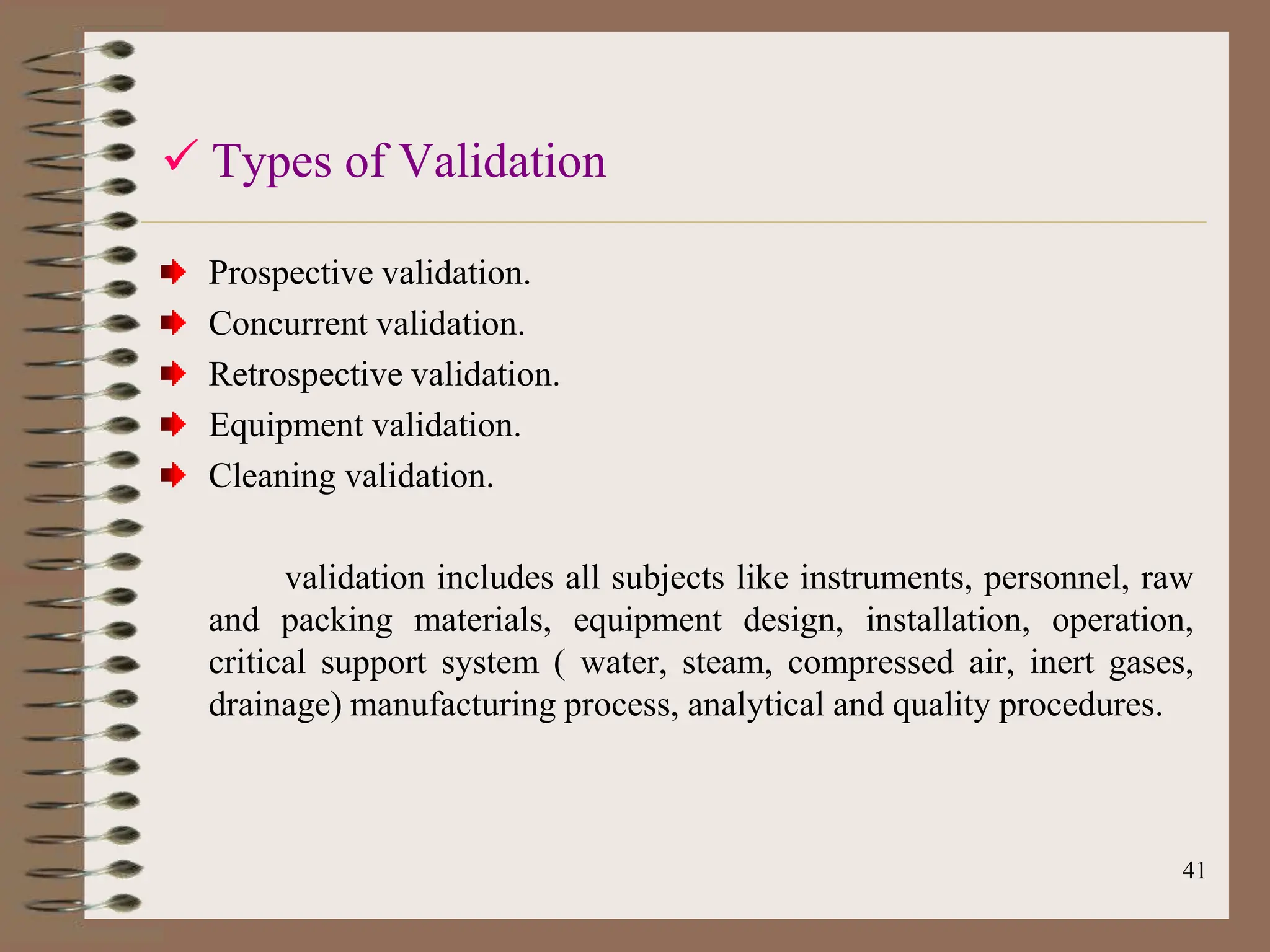
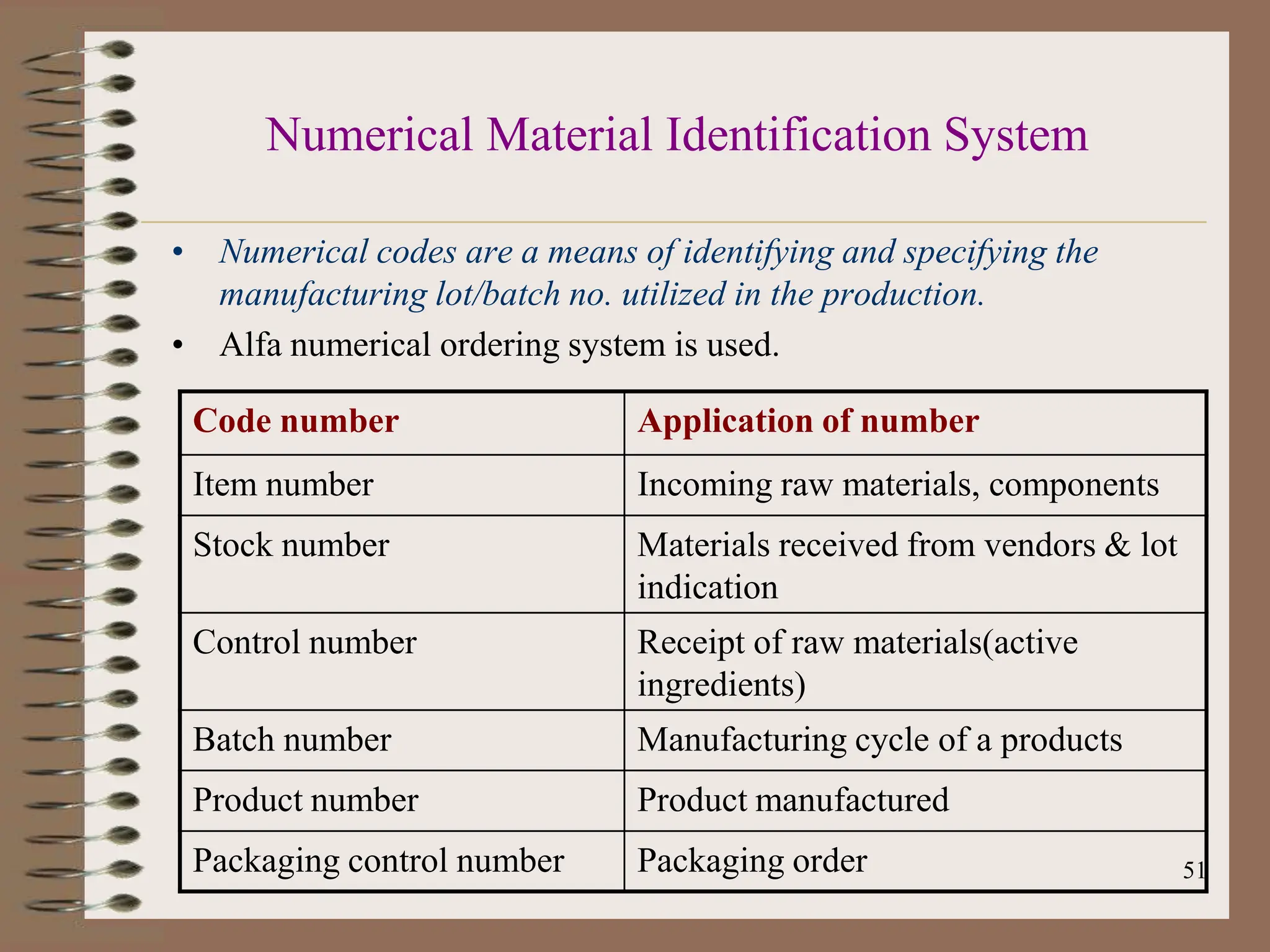
This document provides an overview of Good Manufacturing Practices (GMP), Quality Assurance, and documentation as it relates to the pharmaceutical industry. It discusses the basic concepts of GMP including ensuring quality is built into every stage of manufacturing. It also covers the various aspects regulated by GMP such as personnel, facilities, equipment, materials, and waste disposal. Finally, it emphasizes the importance of documentation to ensure proper procedures are consistently followed.