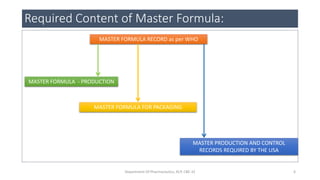
This document discusses various types of documentation required in the pharmaceutical industry, including master formula records (MFR), drug master files (DMF), and generic drug development. It defines MFRs as approved master documents that describe the full manufacturing process for a specific batch size. It provides details on the content required for MFRs based on guidelines from WHO, Health Canada, and the US CFR. It also discusses the purpose and types of DMFs submitted to the FDA, including Type 1 for manufacturing facilities, Type 2 for drug substances/products, and others. Finally, it briefly mentions the Hatch-Waxman Act as it relates to generic drug development.