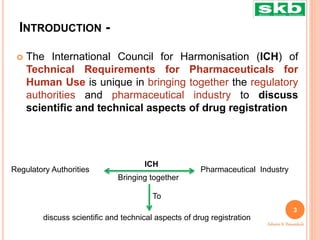
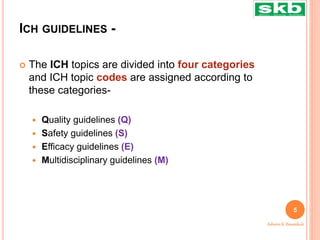
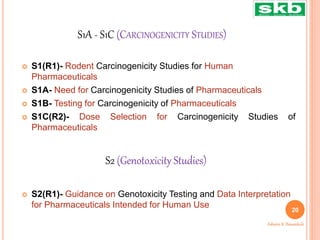
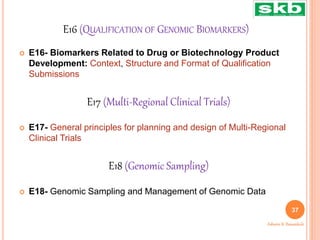
The document discusses guidelines from the International Council for Harmonisation (ICH) related to quality, safety, efficacy, and multidisciplinary aspects of pharmaceutical development. It provides an overview of ICH guidelines in four categories (Q, S, E, M) and describes some of the key guidelines within the Quality (Q) and Safety (S) categories in more detail. The Quality guidelines address issues like stability testing, impurities, biotechnology products, and good manufacturing practices. The Safety guidelines cover topics such as carcinogenicity, genotoxicity, reproductive toxicity, and non-clinical safety evaluation.