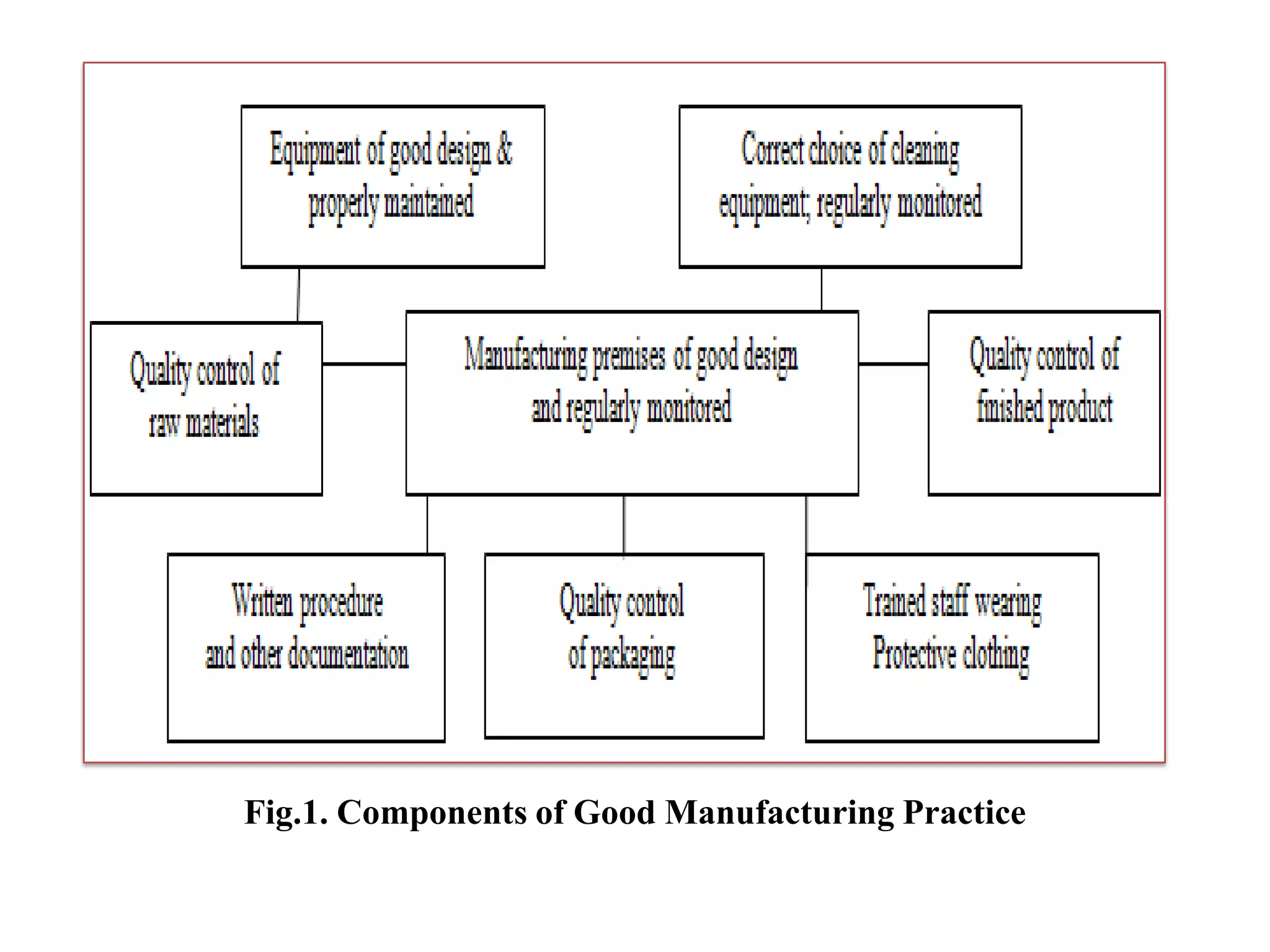
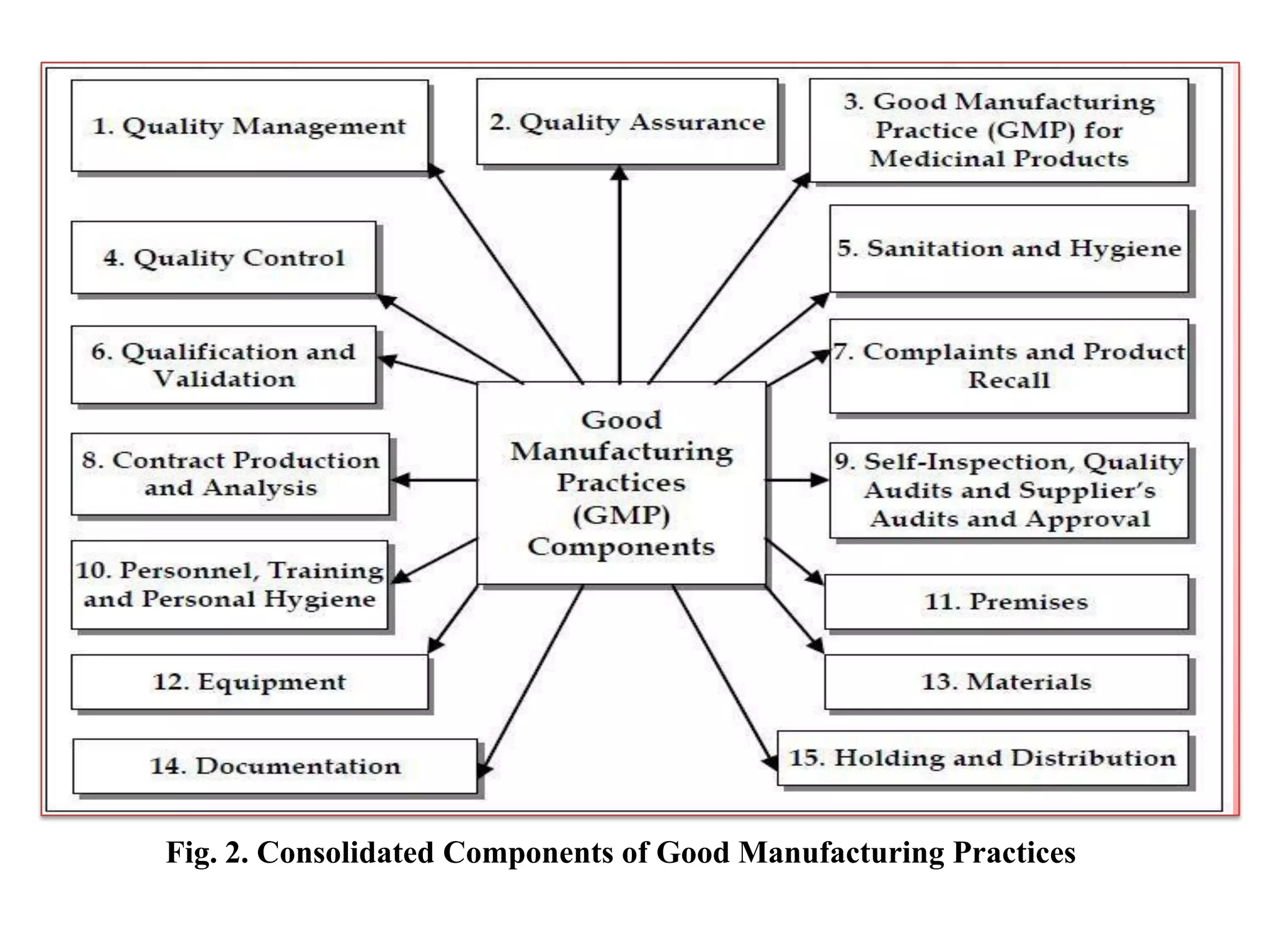
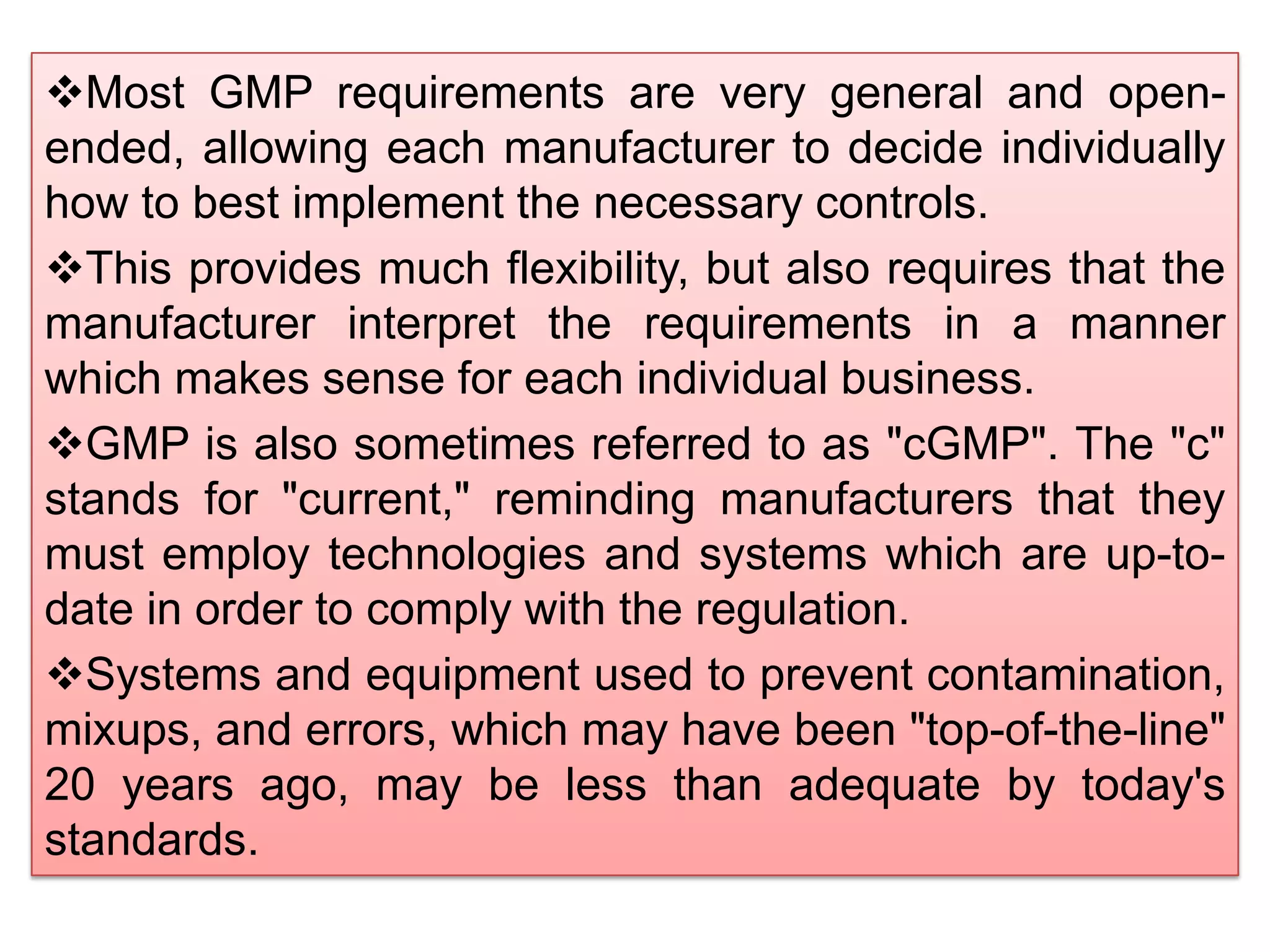
Good Manufacturing Practice (GMP) is a regulatory system ensuring consistent production and quality control of pharmaceutical products to minimize risks through comprehensive procedures, documentation, and training. It encompasses all elements of production, including equipment, personnel hygiene, and quality assurance, and non-compliance can lead to serious legal consequences. GMP regulations remain broadly defined, allowing flexibility for manufacturers to implement necessary controls while highlighting the importance of current practices to maintain safety and efficacy.