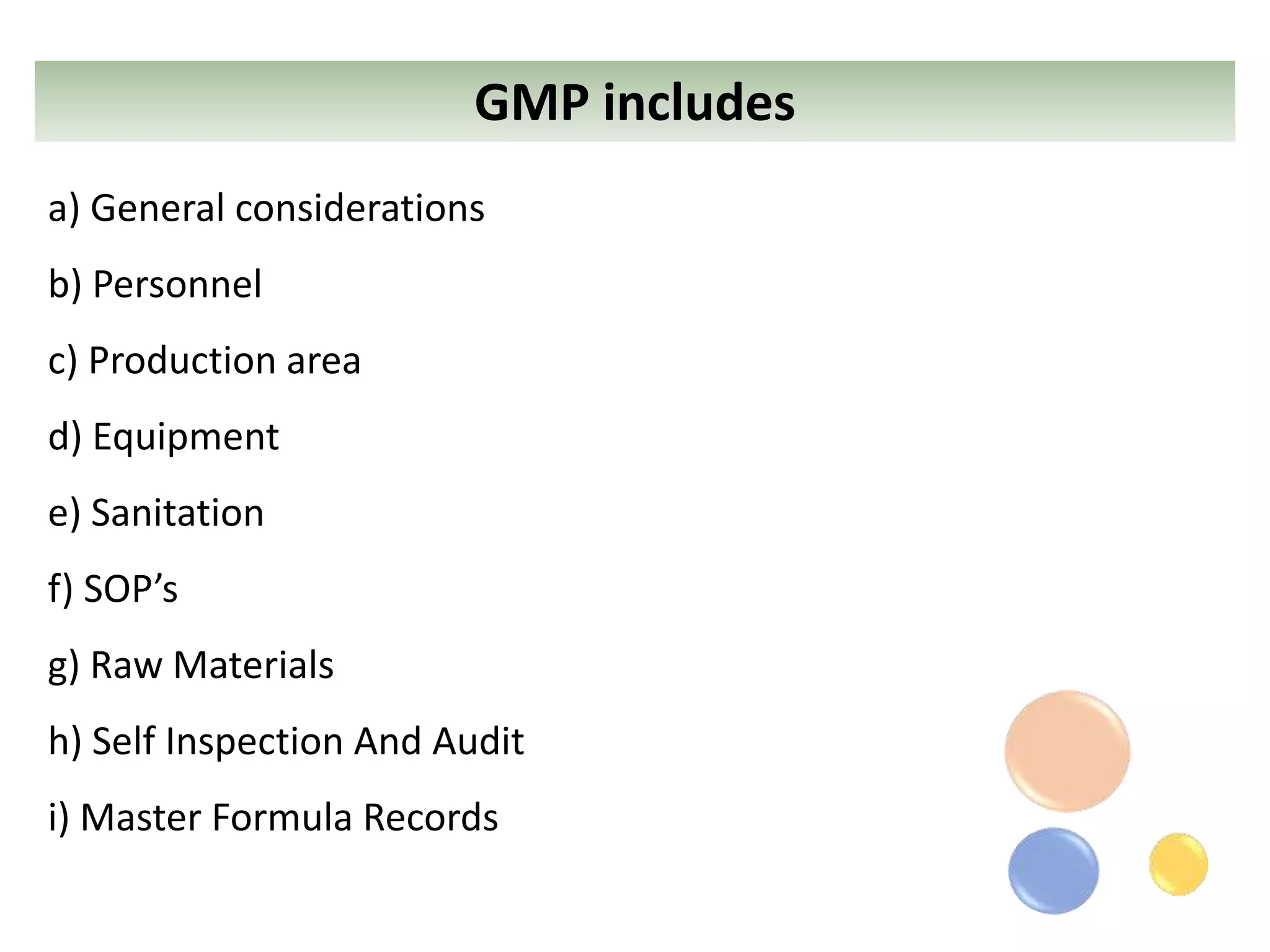
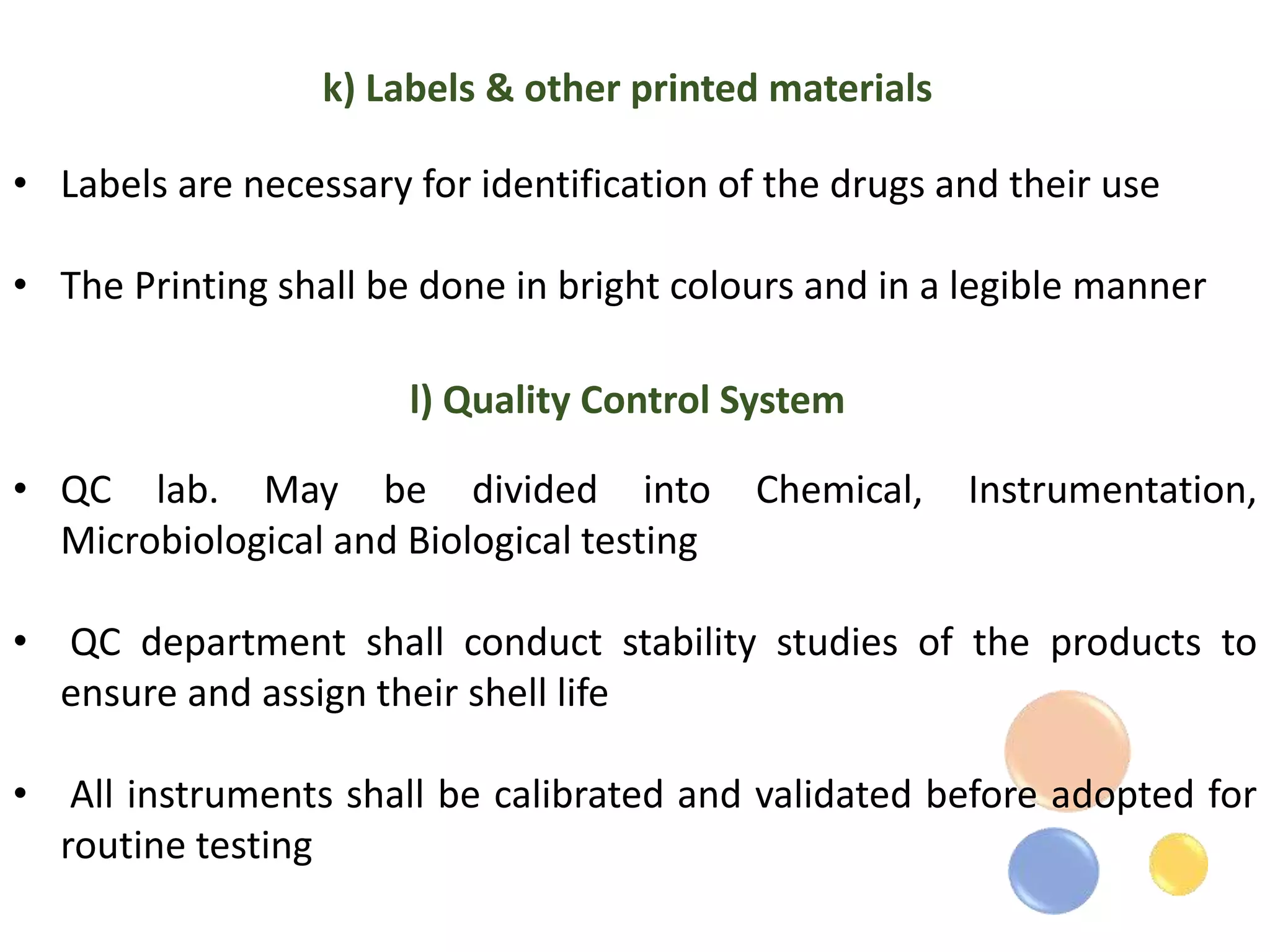
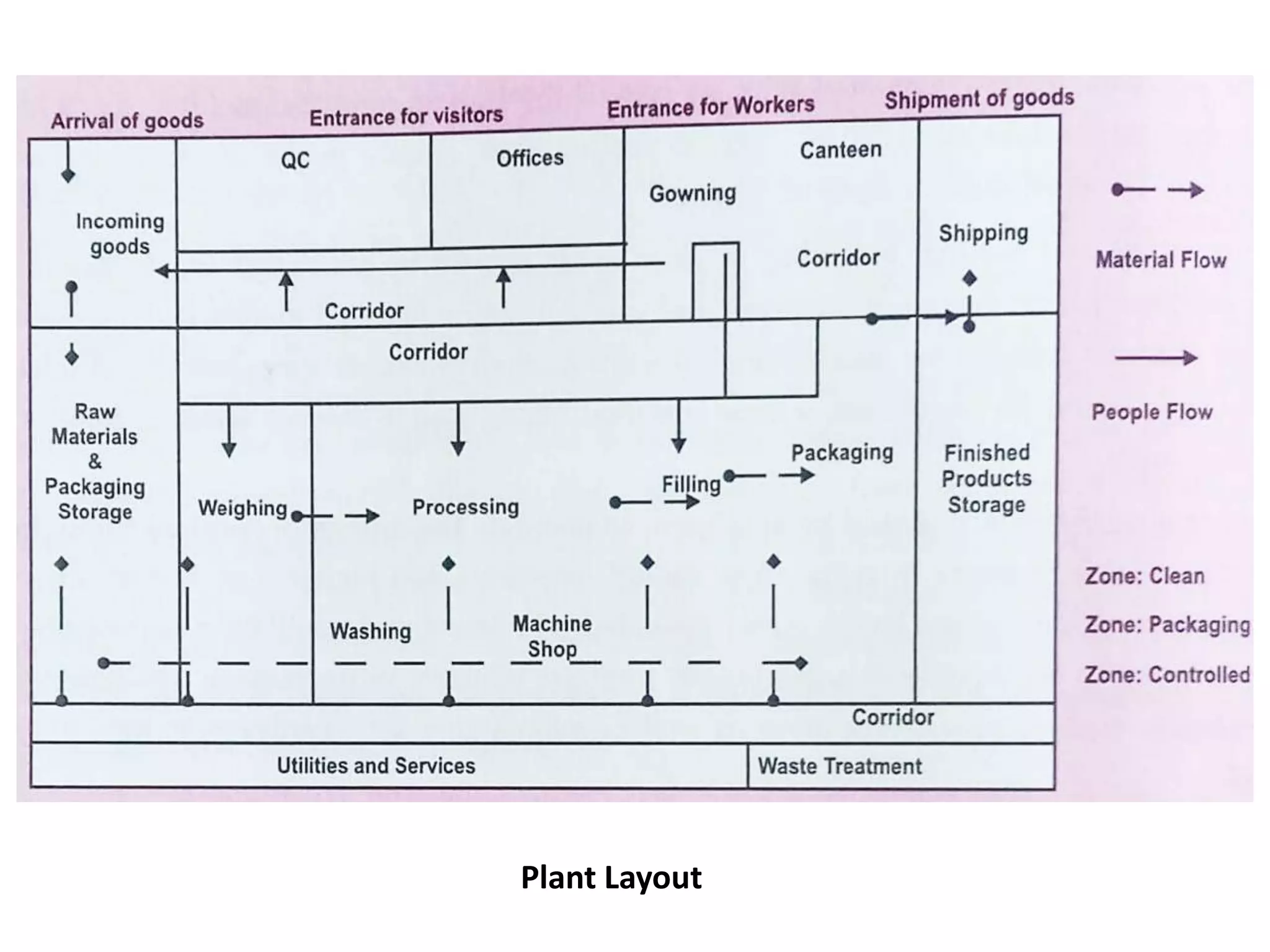
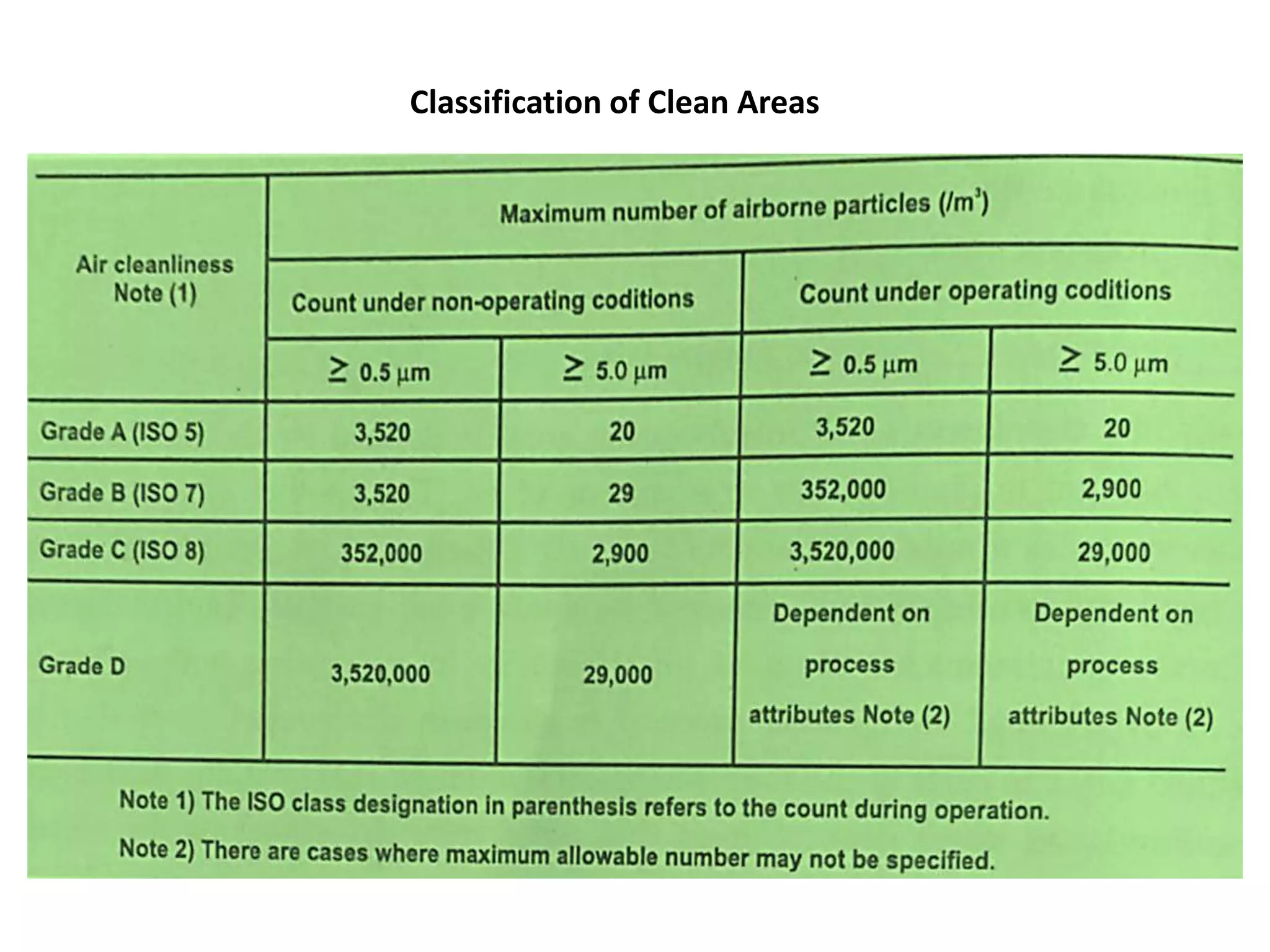
The document outlines the current Good Manufacturing Practices (cGMP) guidelines as defined by various organizations including the FDA and WHO, emphasizing the importance of maintaining quality and reducing risks in pharmaceutical manufacturing. It details necessary considerations for facilities, personnel, equipment, and quality control systems, as well as environmental monitoring to prevent contamination. Additionally, the document references regulations from 21 CFR and international agreements aimed at harmonizing GMP standards across countries.