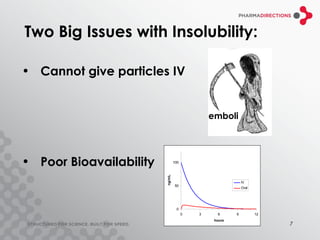
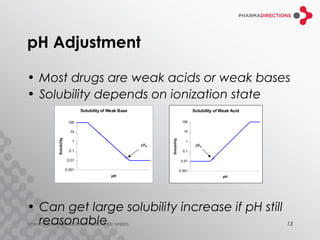
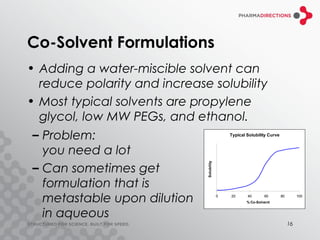
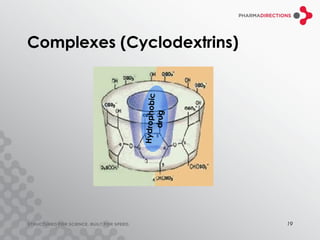
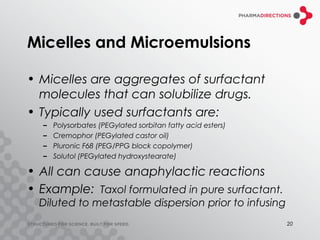
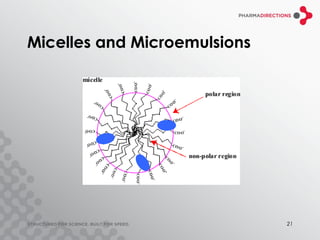
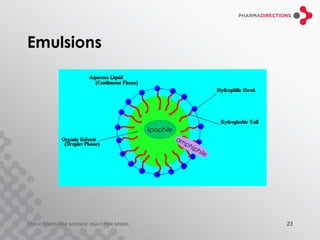
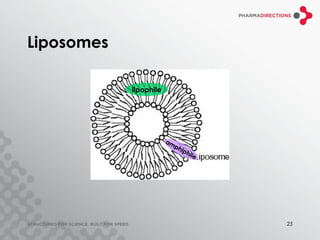
The document discusses the challenges of formulating poorly soluble drugs, emphasizing that as new drugs become increasingly insoluble, med chemists may overlook solubility despite its critical importance. It explores various strategies to enhance bioavailability such as adjusting pH, using co-solvents, and employing complexation techniques like cyclodextrins and micelles. Additionally, it highlights the complexities involved in choosing the right formulation based on factors like drug stability, administration route, and regulatory considerations.