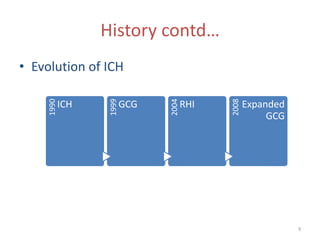
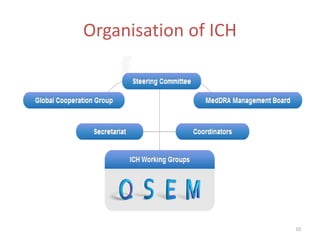
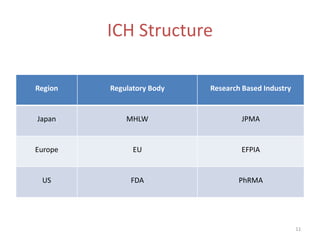
The International Conference on Harmonisation (ICH) was created in 1990 as a unique effort between regulators and industry from the EU, Japan, and US to harmonize technical requirements for pharmaceutical registration. ICH aims to ensure safety, efficacy, and quality of medicines while preventing duplicative trials and minimizing animal testing. Through guidelines developed via consensus building among members, ICH has harmonized requirements for drug development and approval processes. However, some concerns remain regarding inclusion of non-members in the decision making and implications for developing countries.