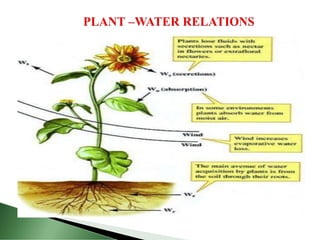
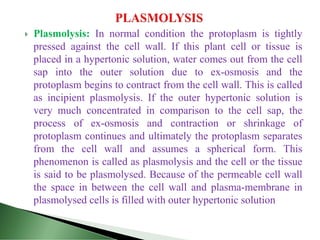
This document provides information about plant water relations and the absorption of water by plant roots. It discusses that water is essential for plant life and is absorbed by root hairs from the soil. Root hairs enter the spaces between soil particles and absorb water through a process of osmosis, facilitated by their selectively permeable cell membranes. Water then moves through the plant, powering processes like photosynthesis and supporting plant structure through turgor pressure in cells.