1) Biophysics is the science that explains biological systems and processes using the principles of physics. It spans scales from molecules to whole organisms.
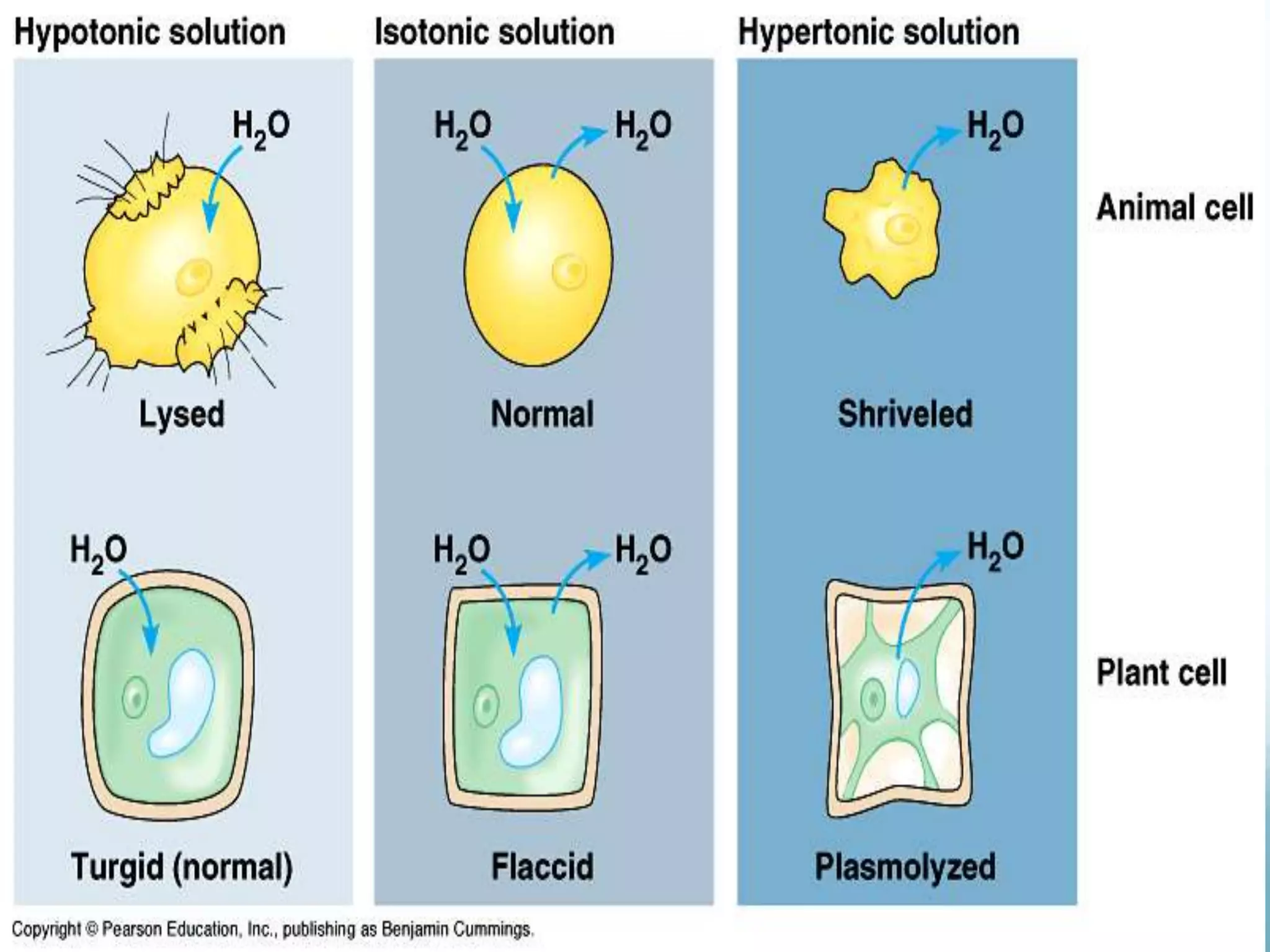
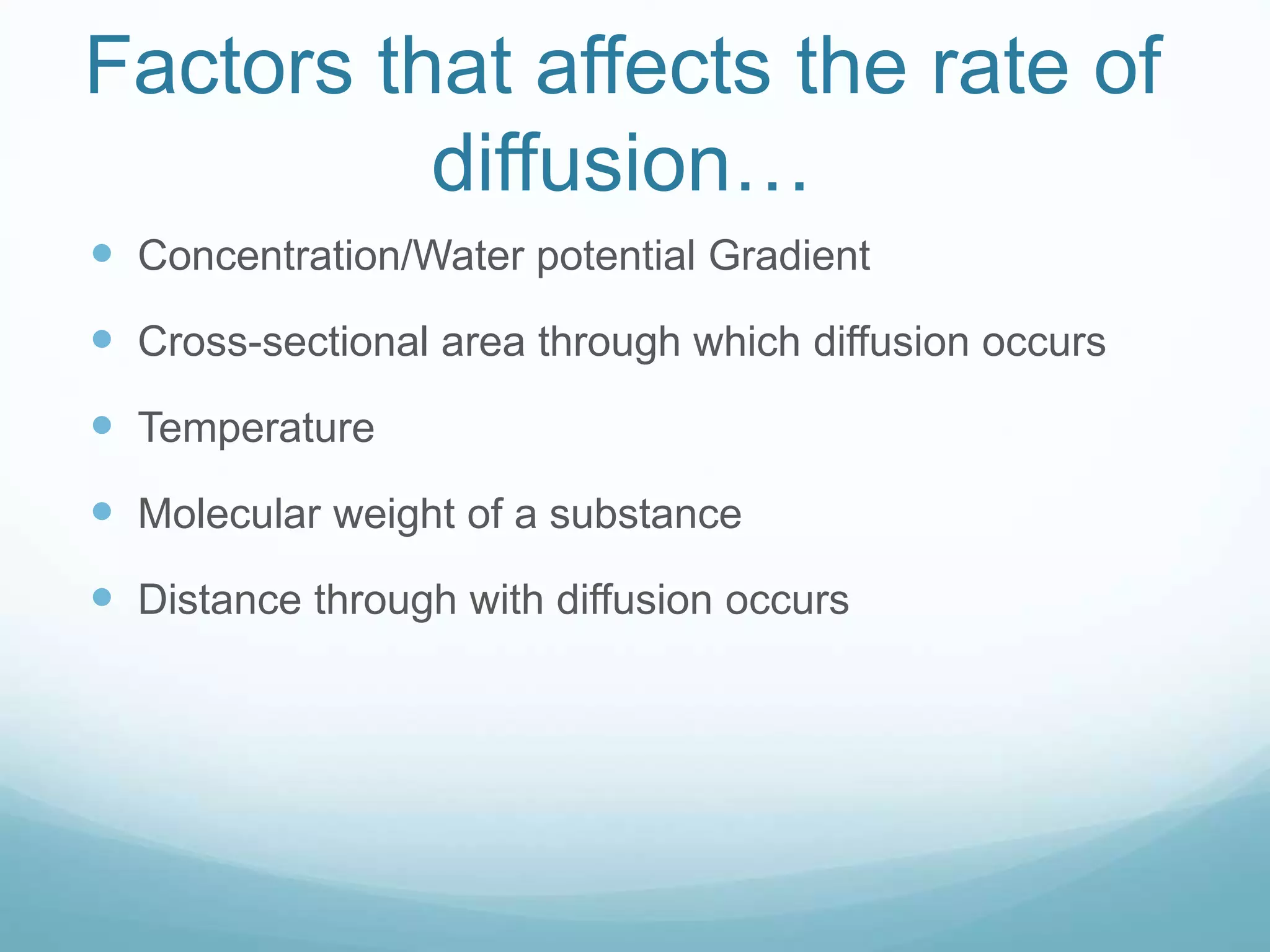
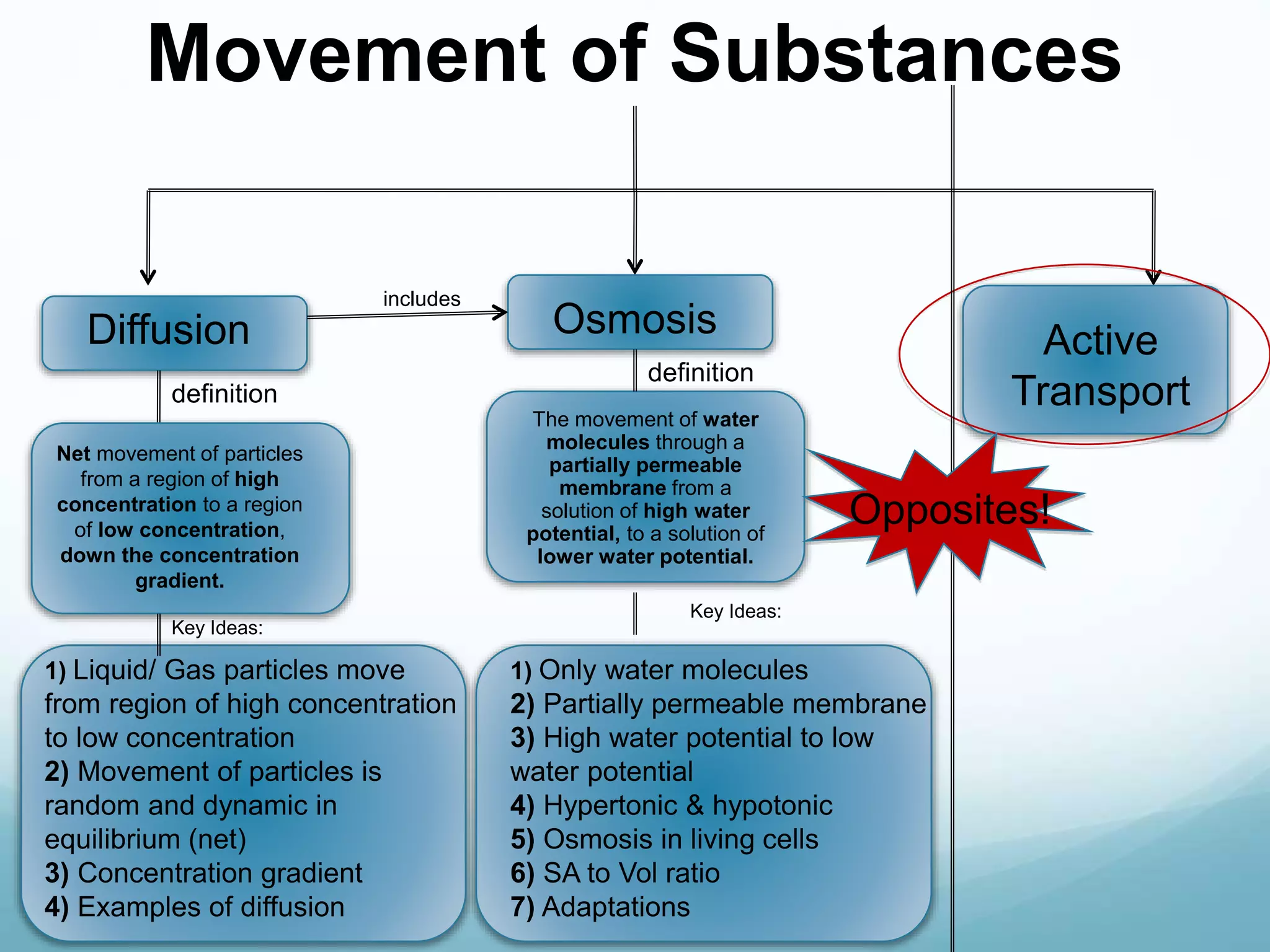
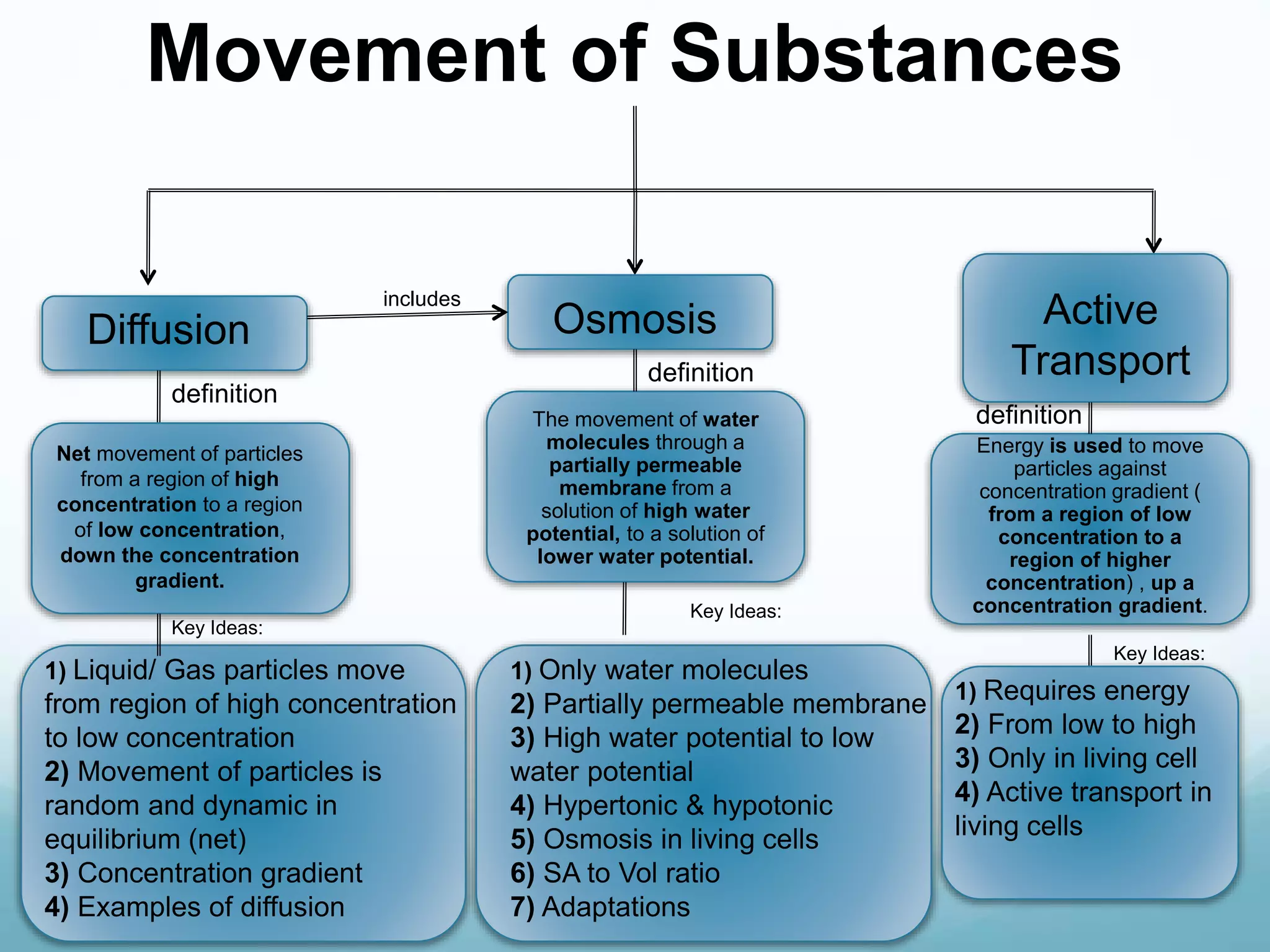
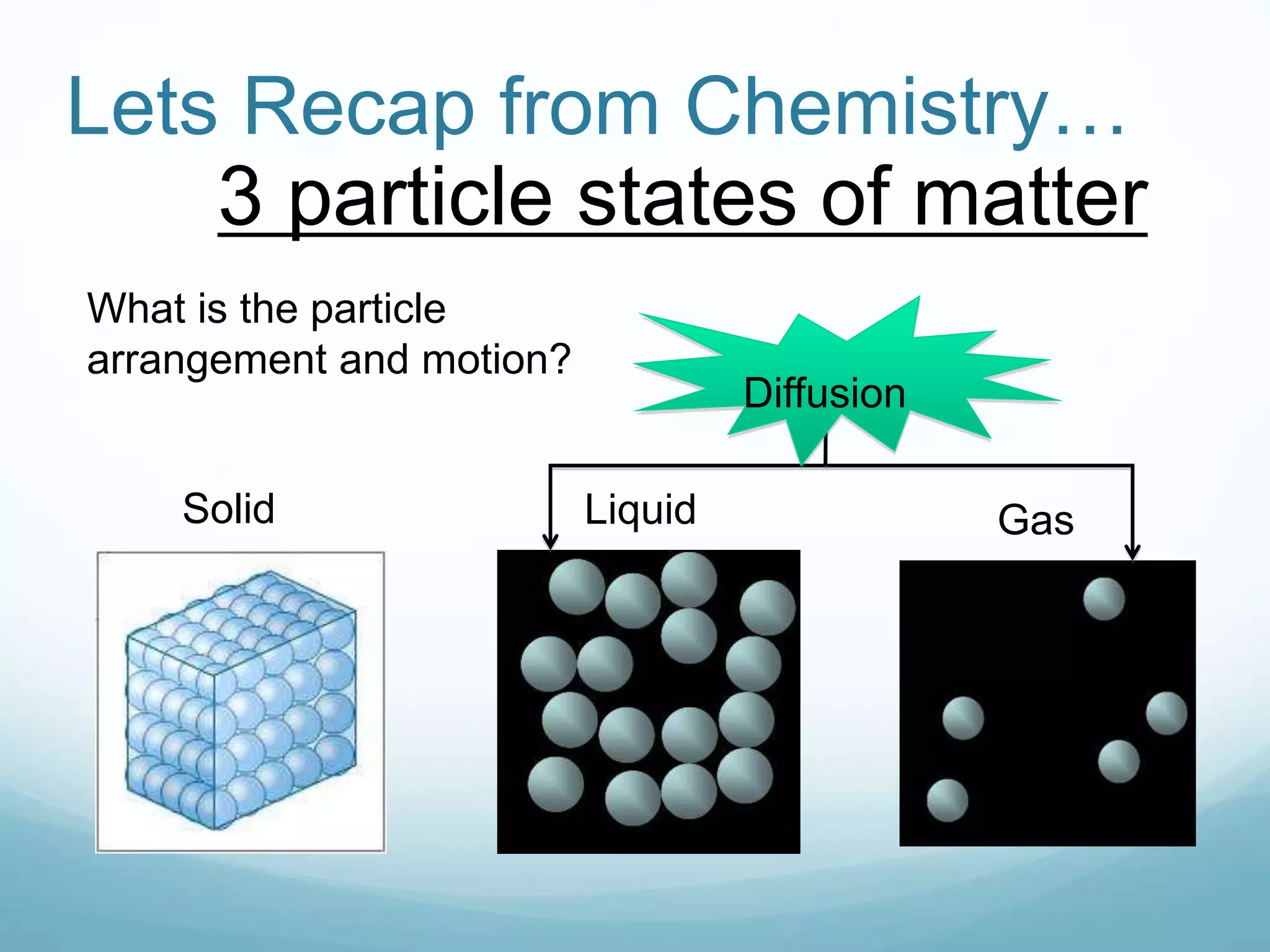
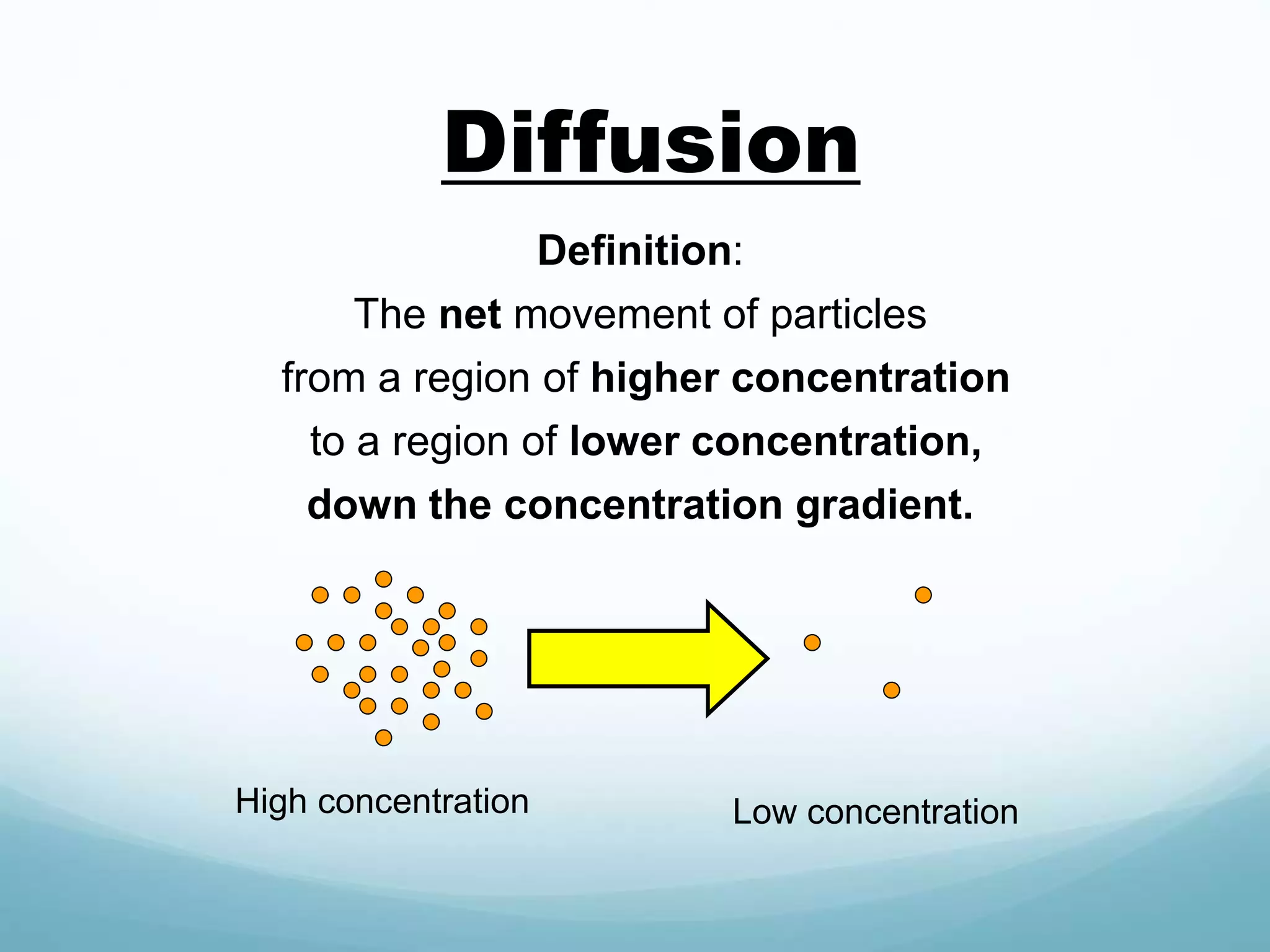
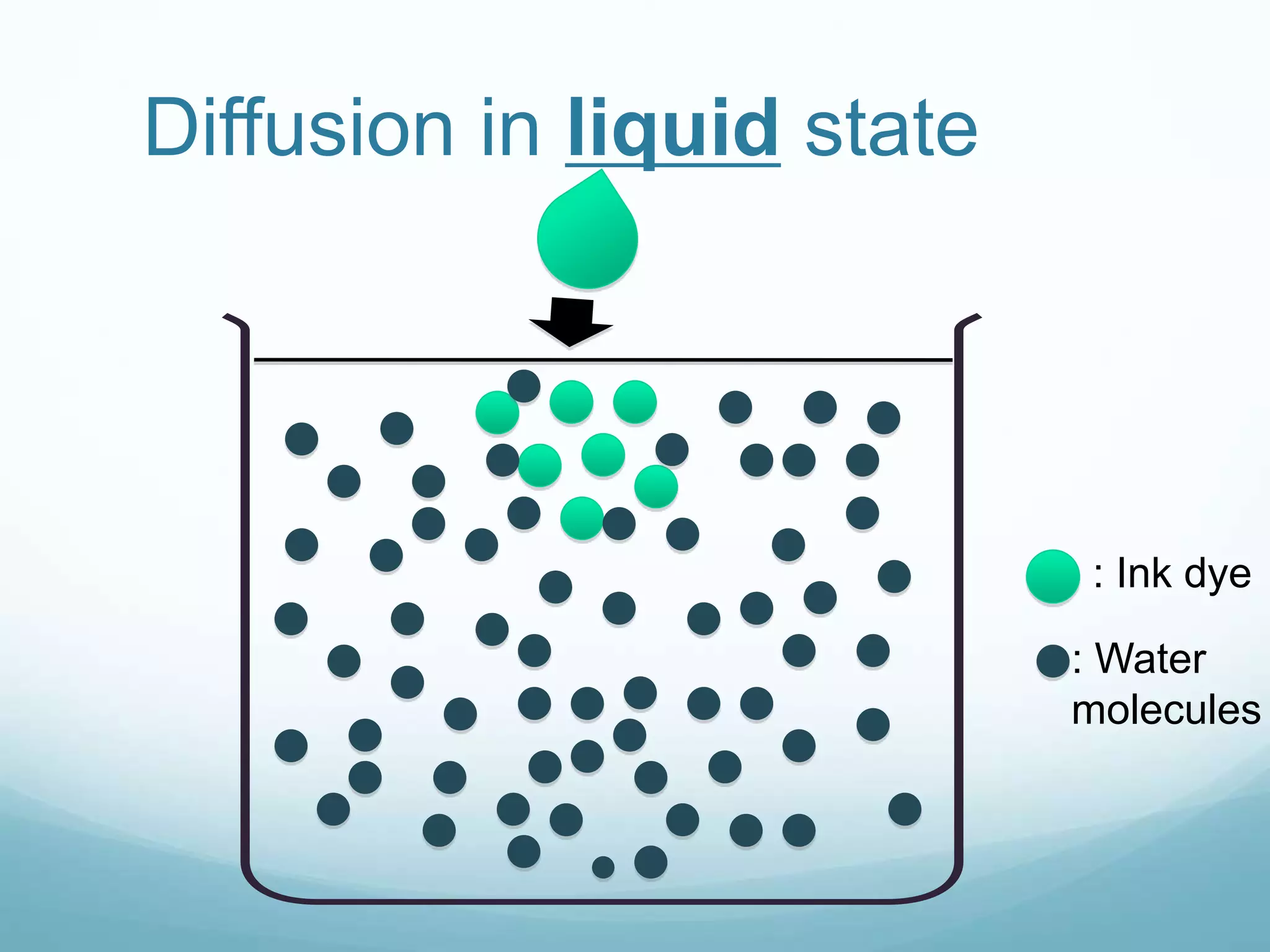
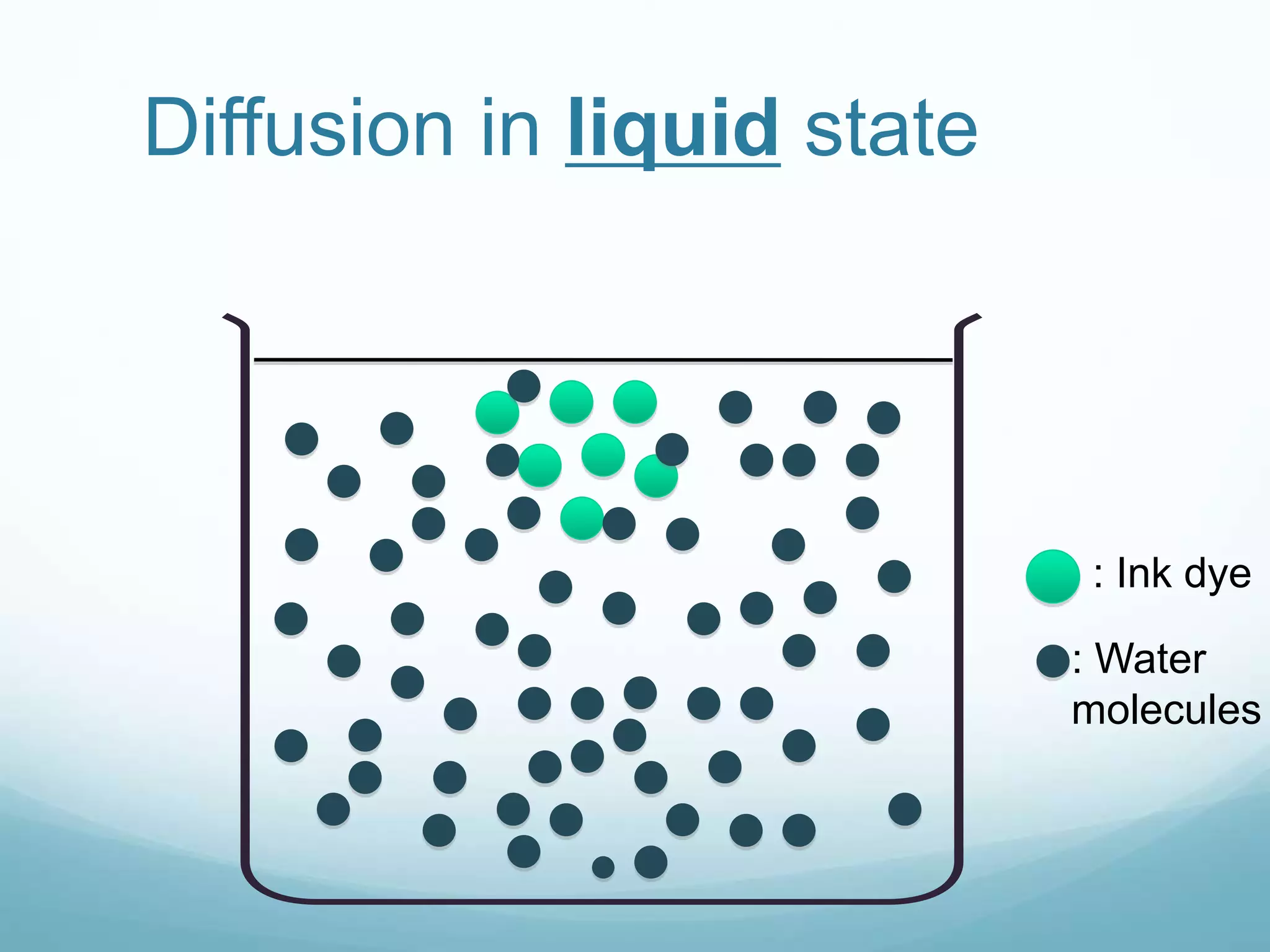
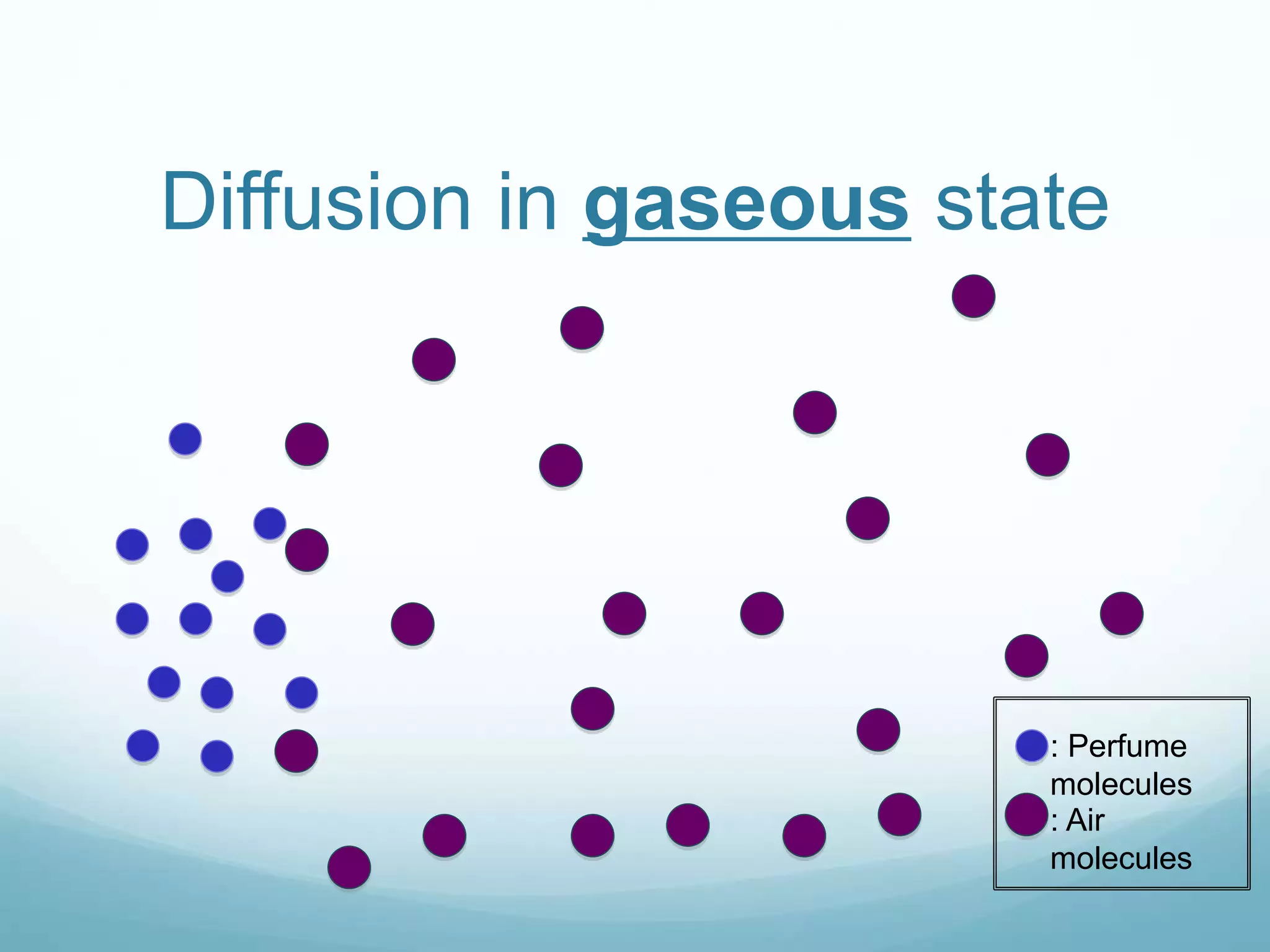
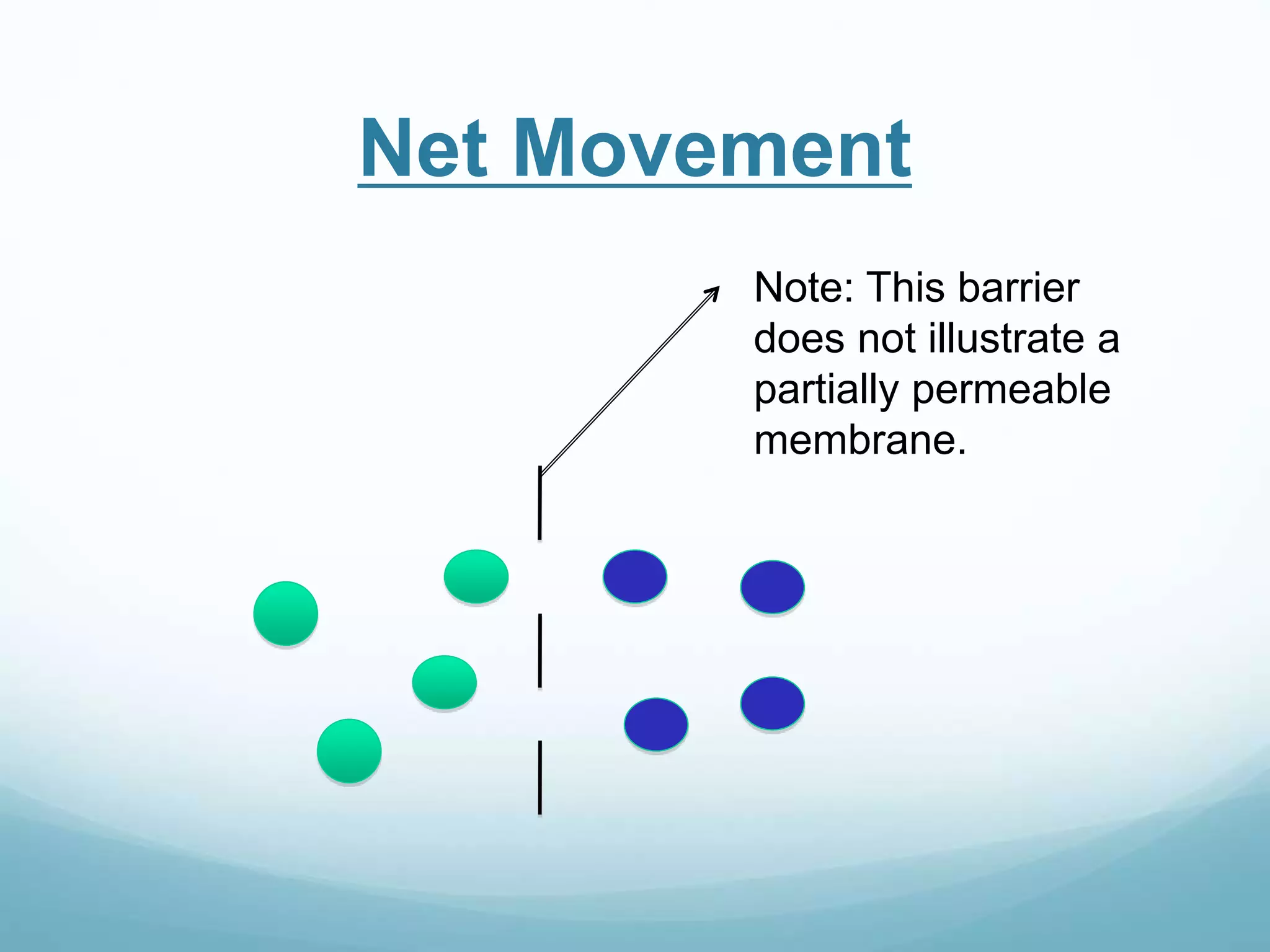
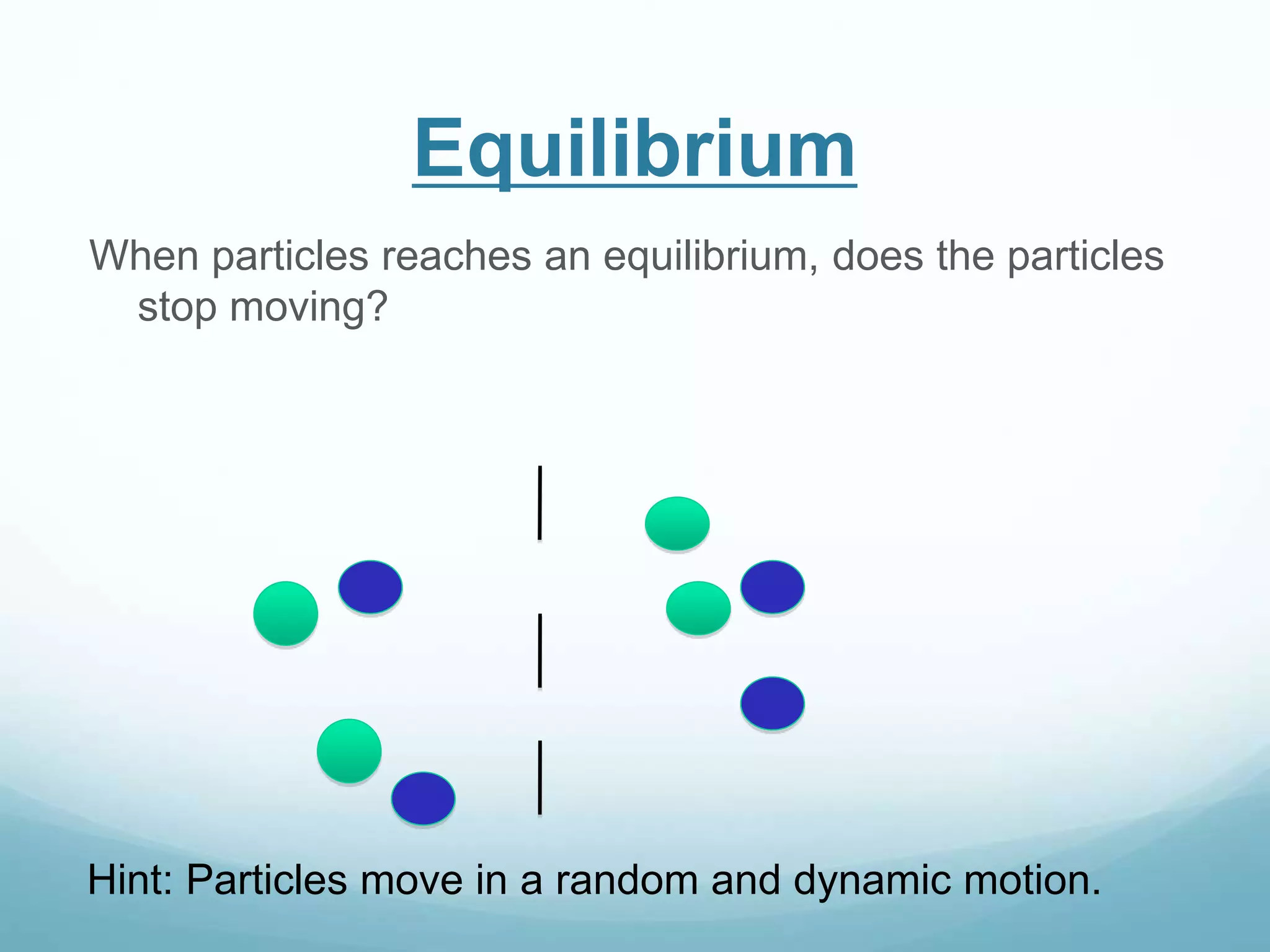
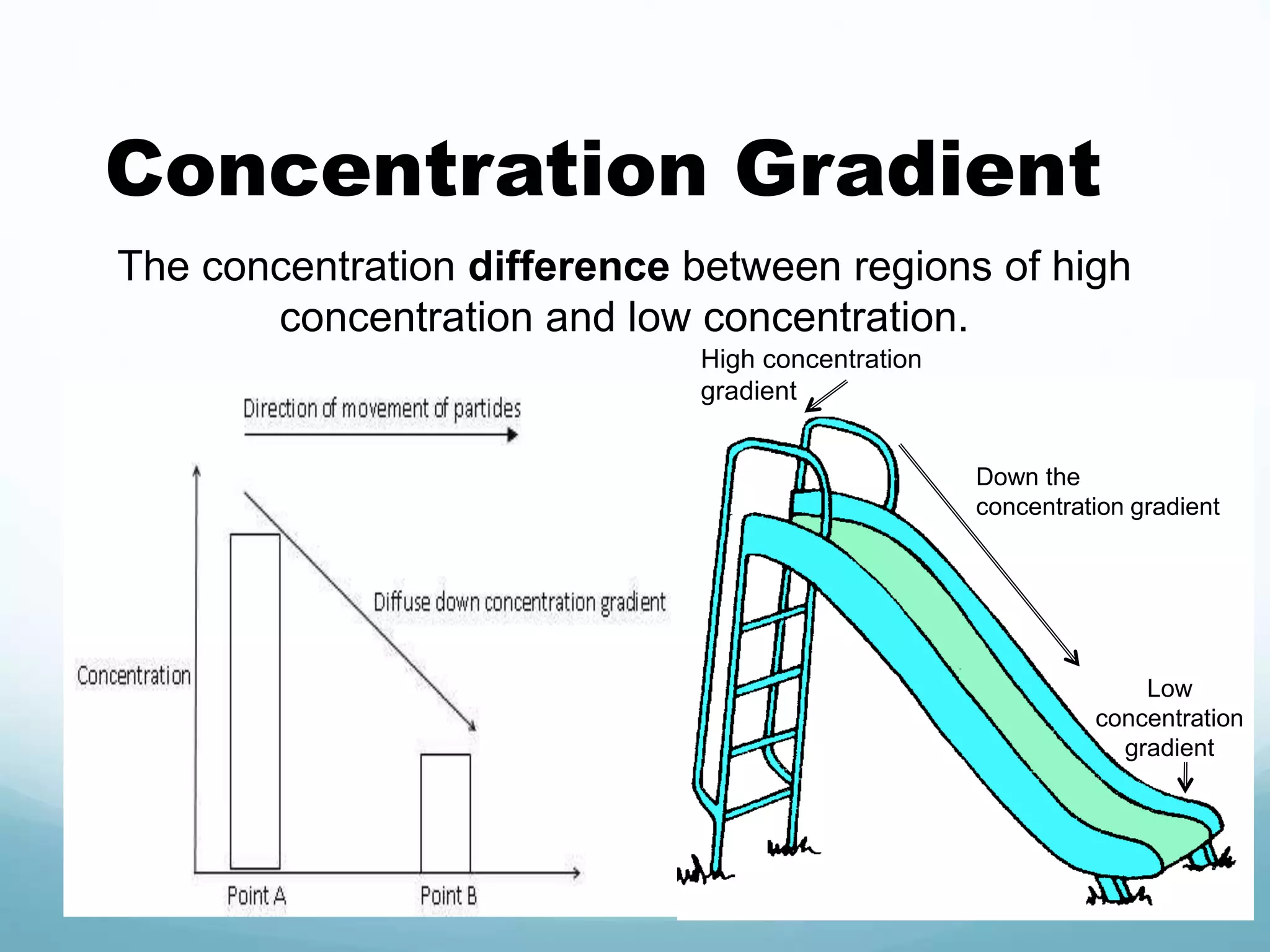
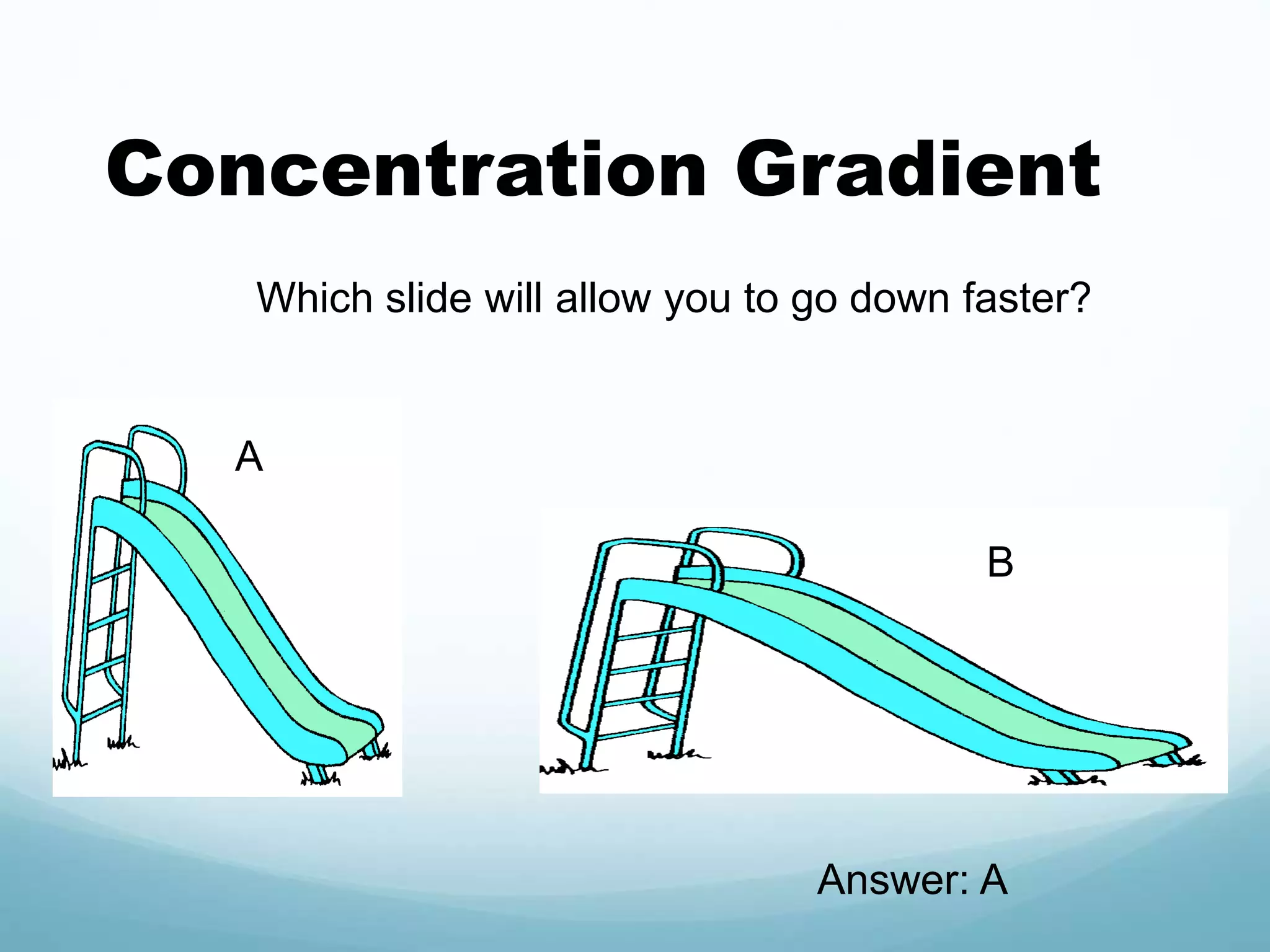
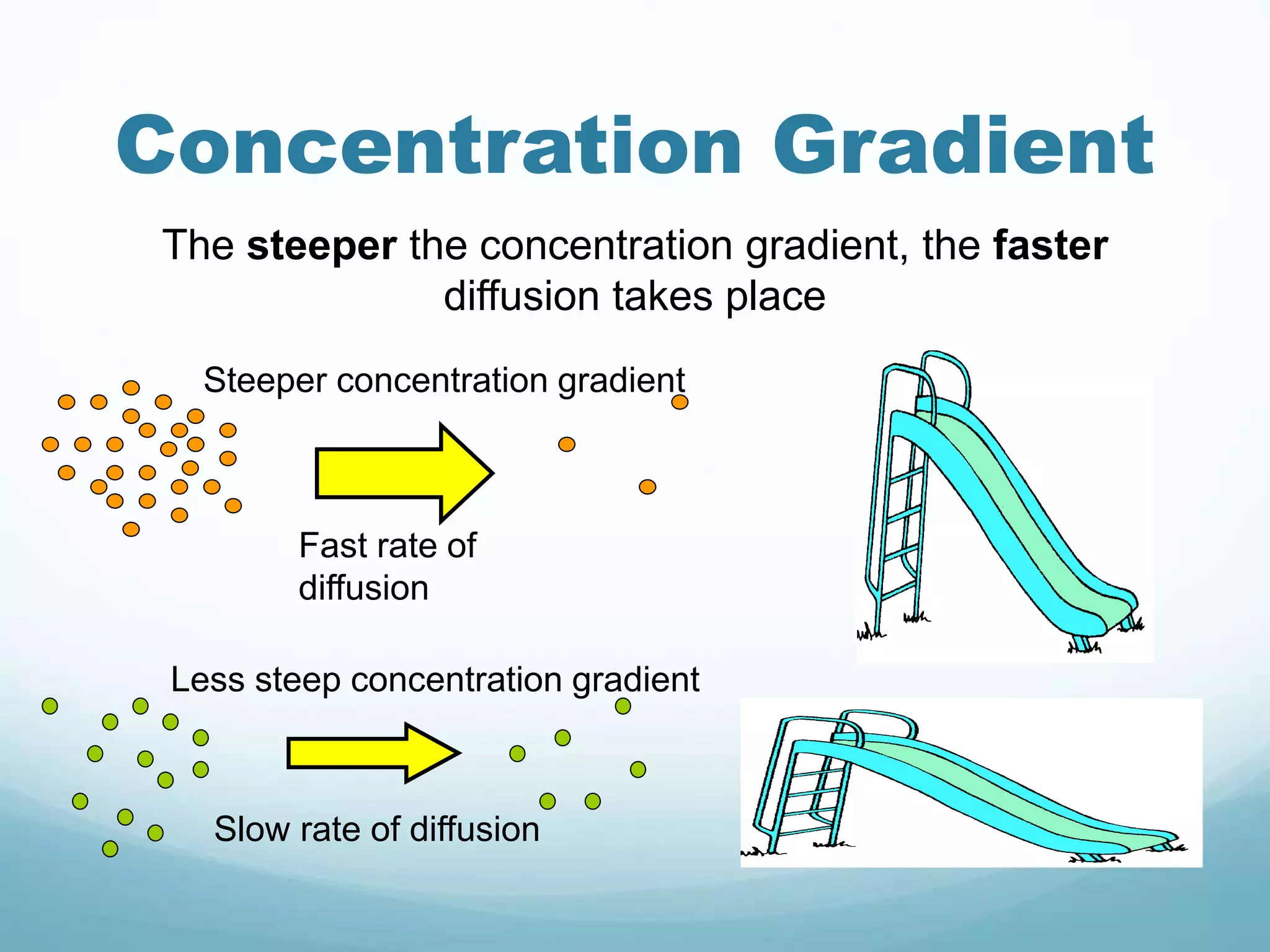
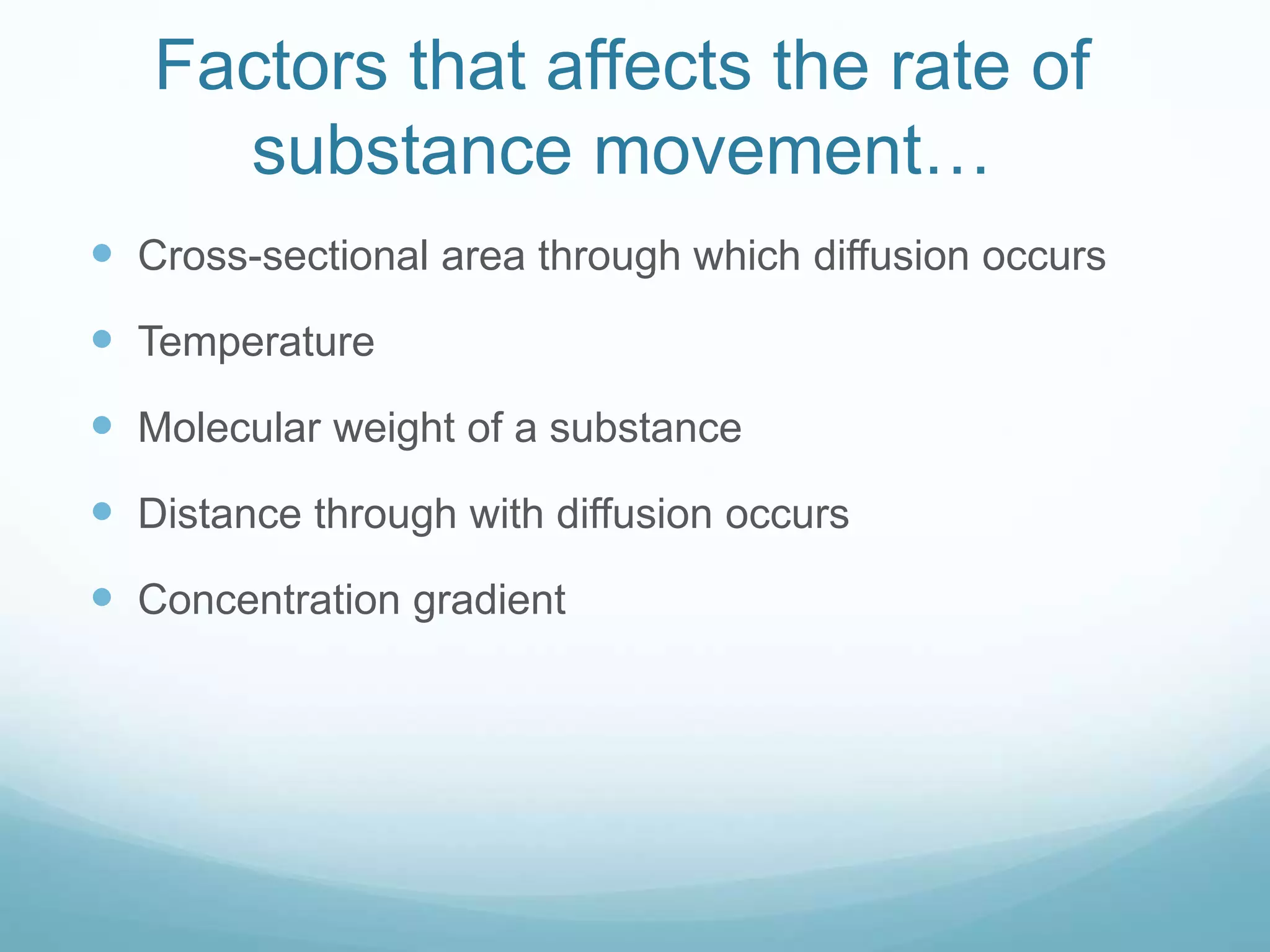
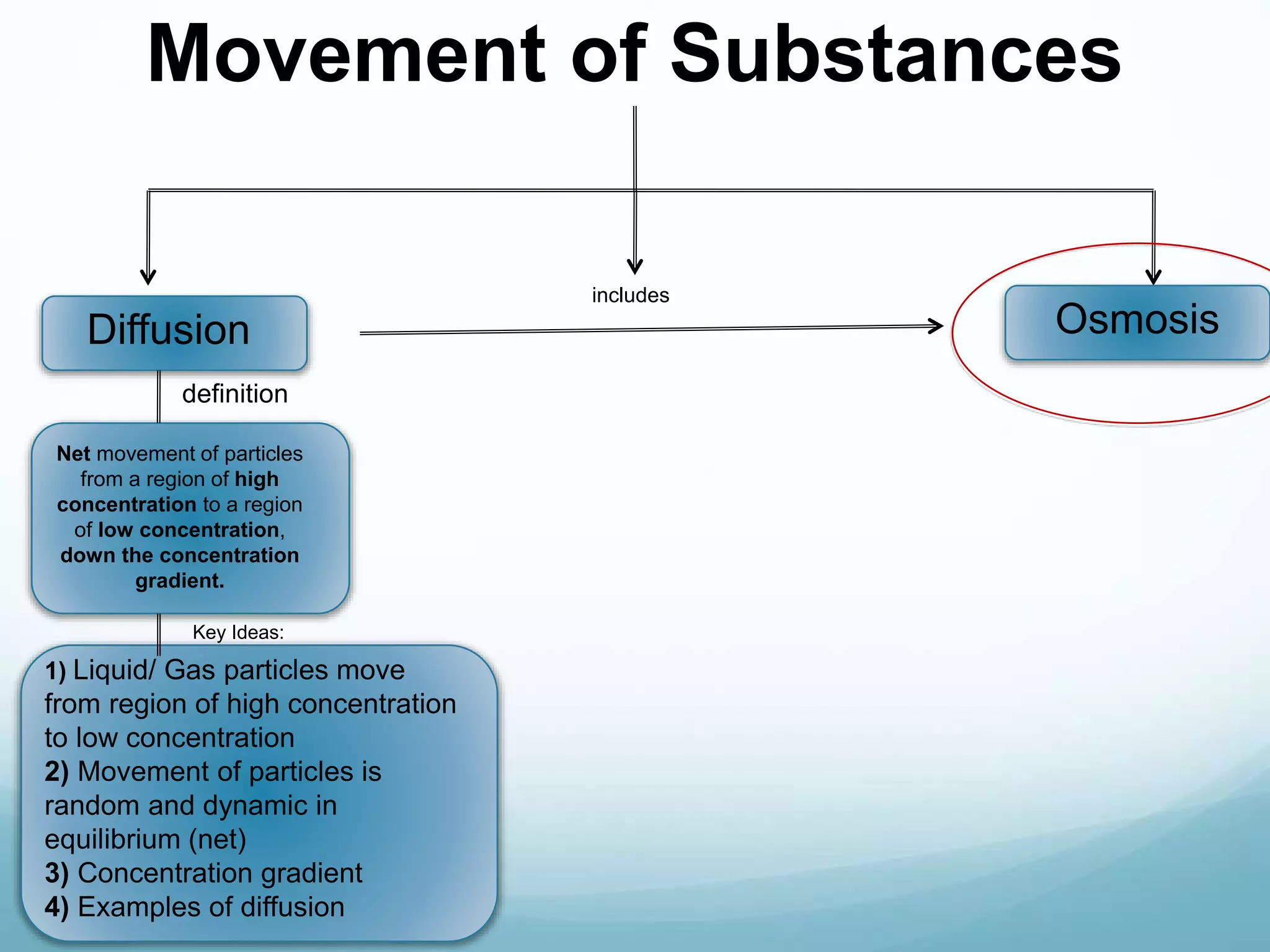
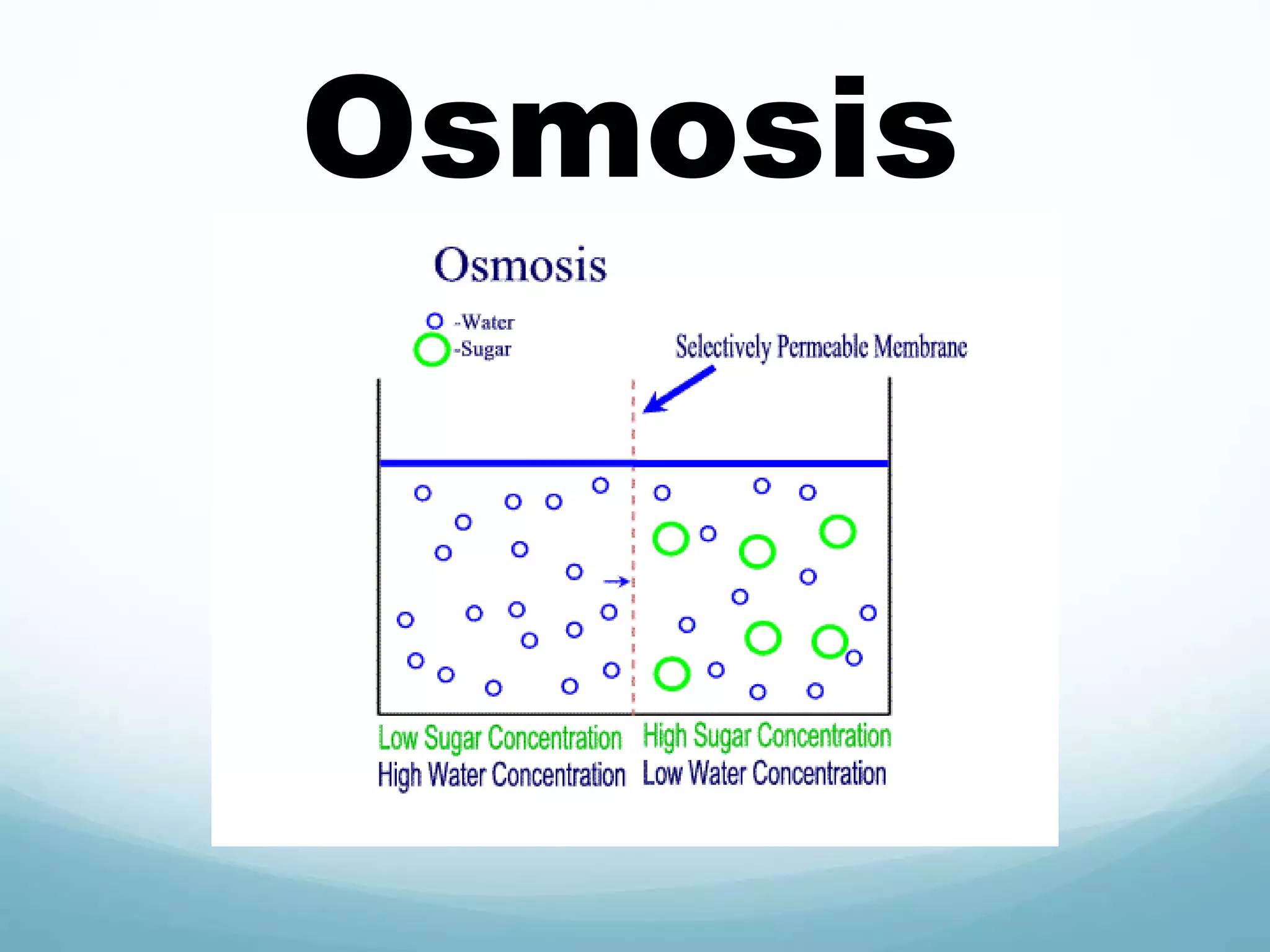
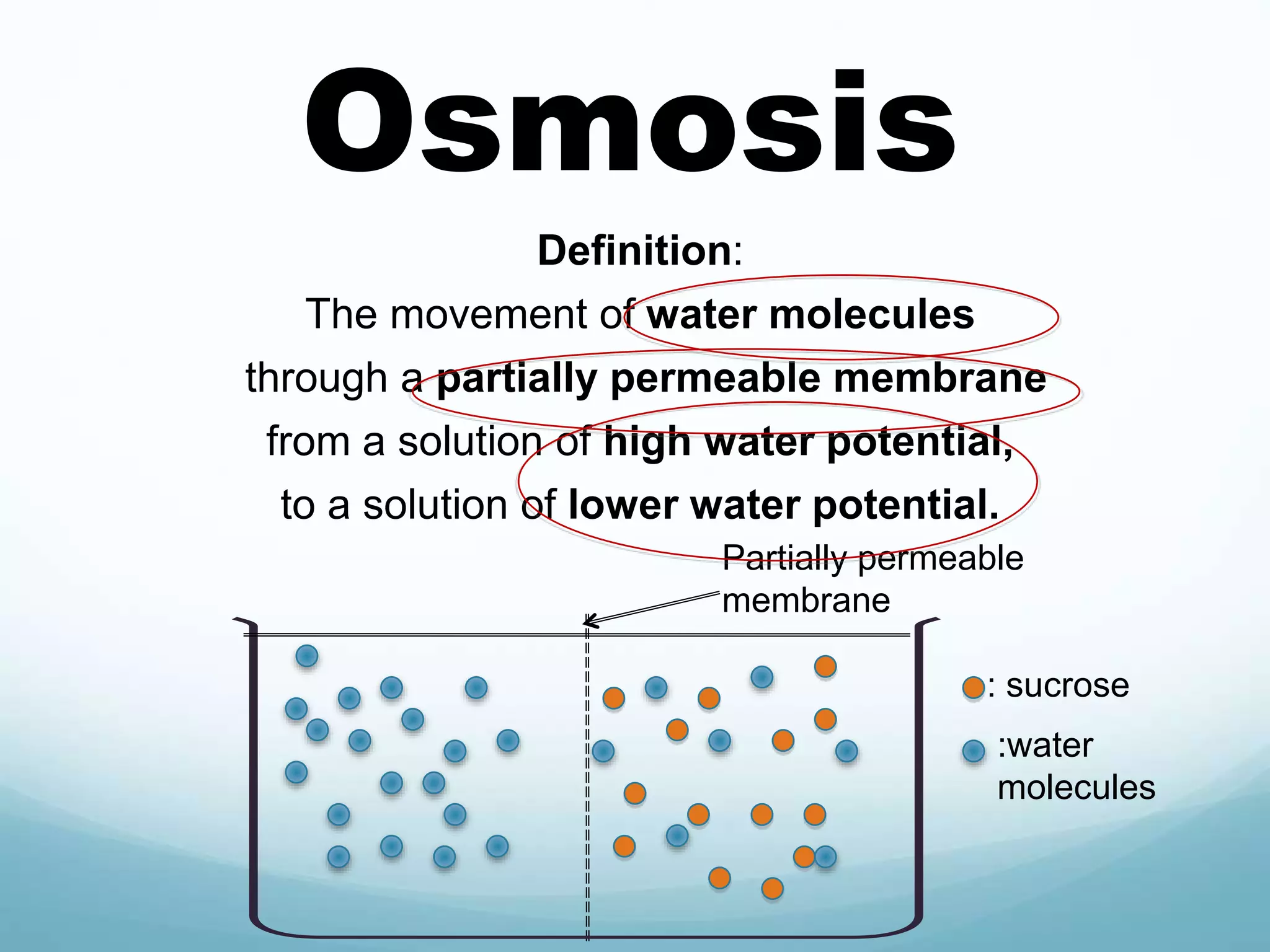
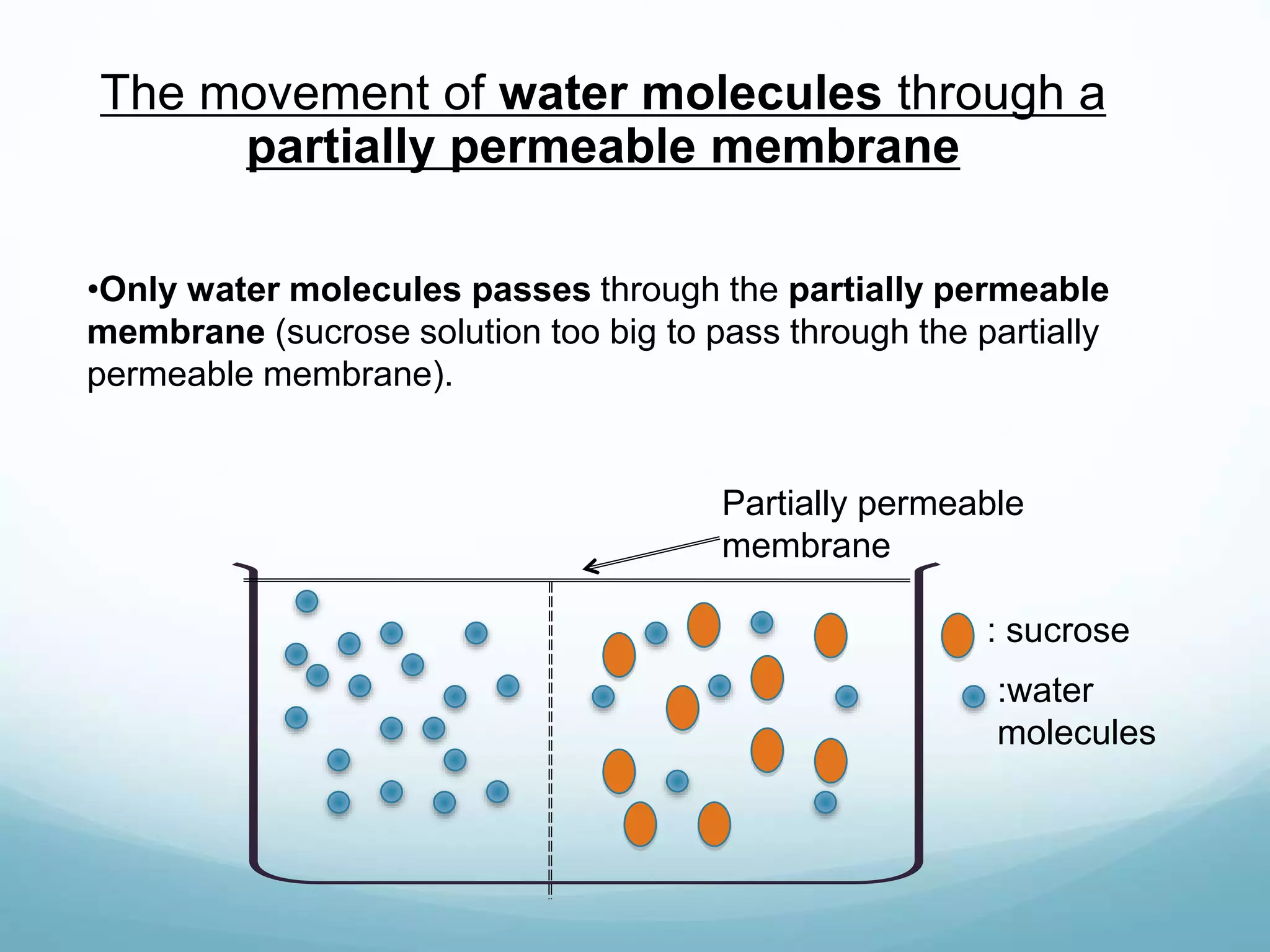
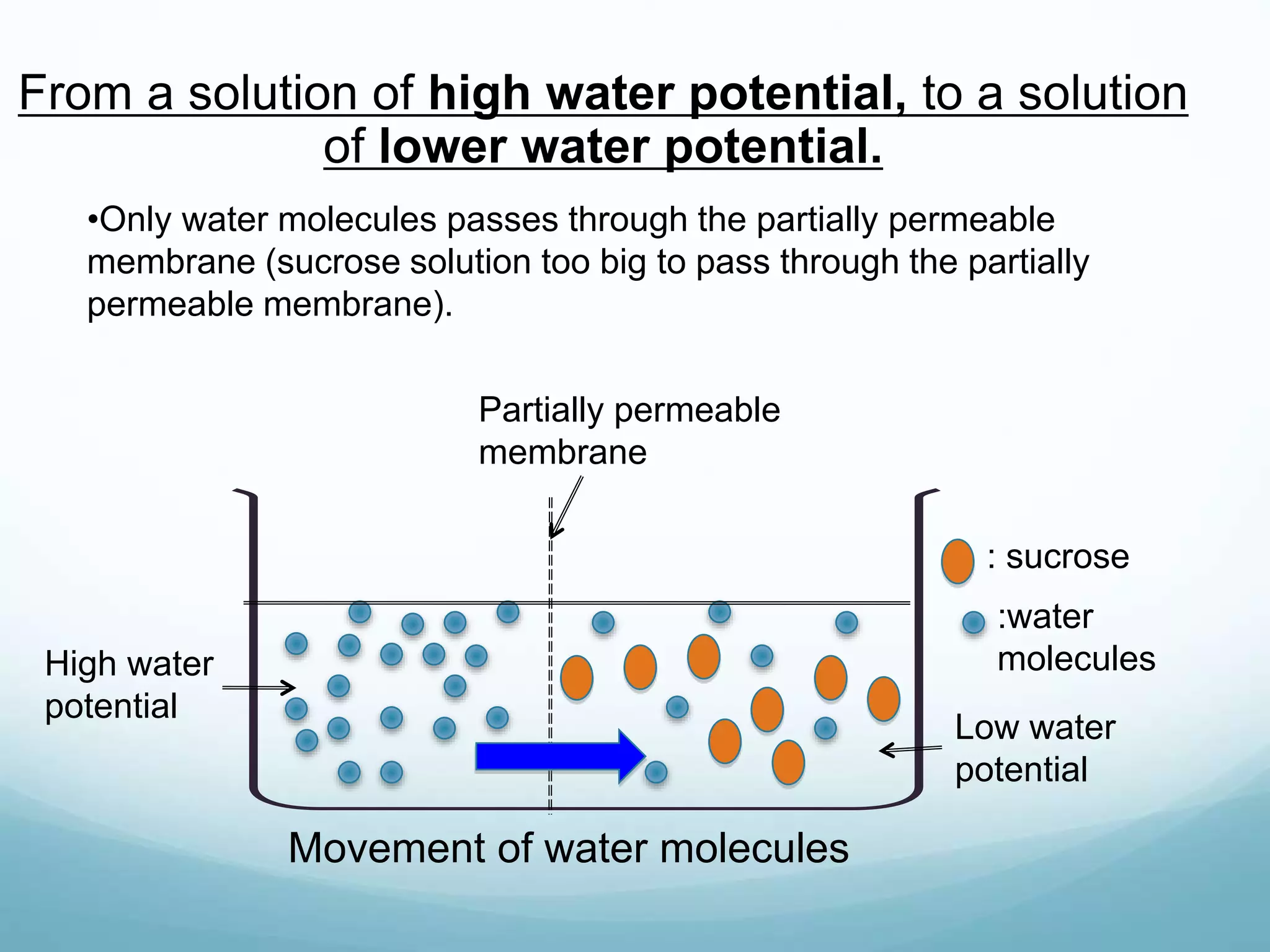
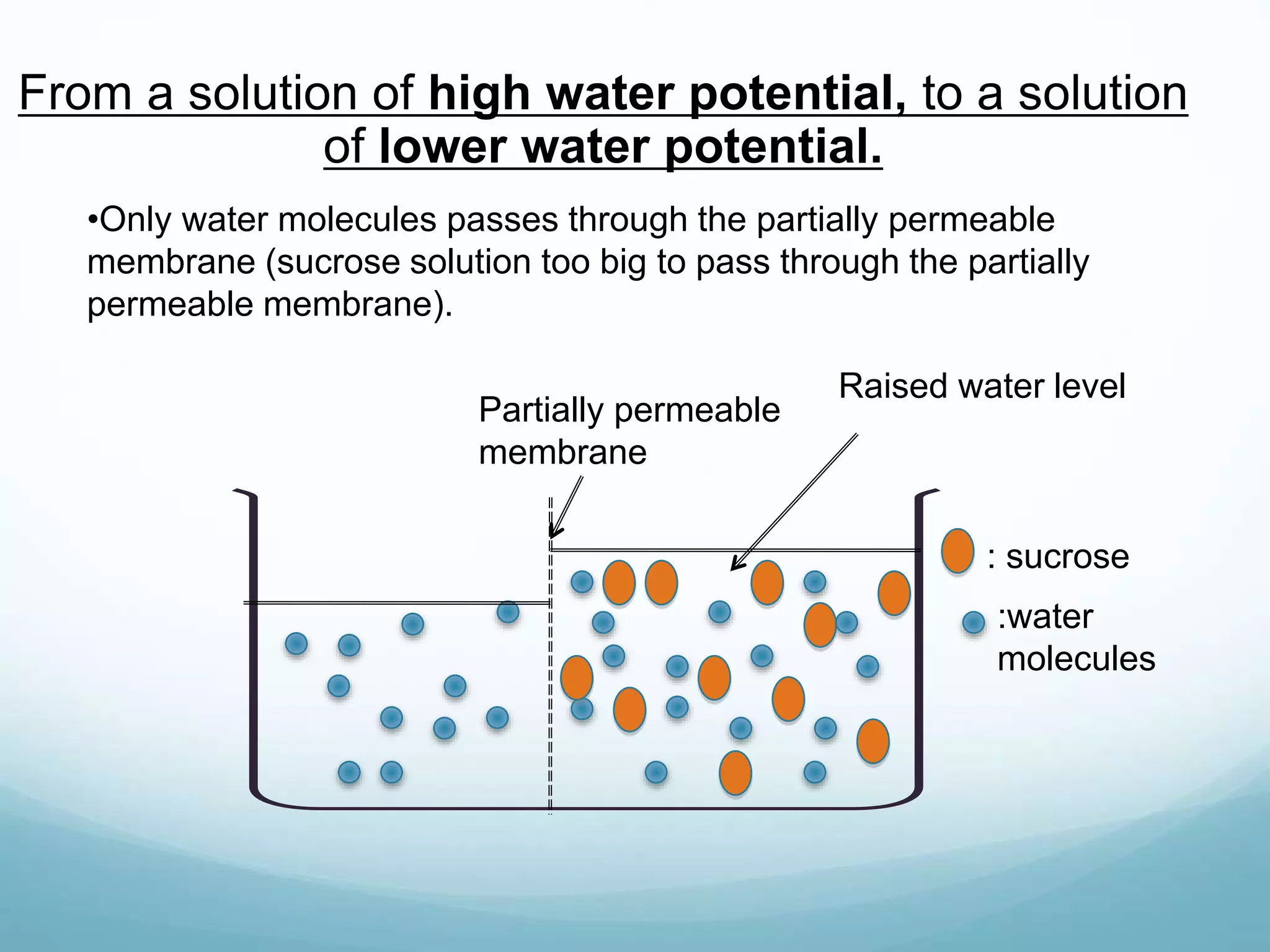
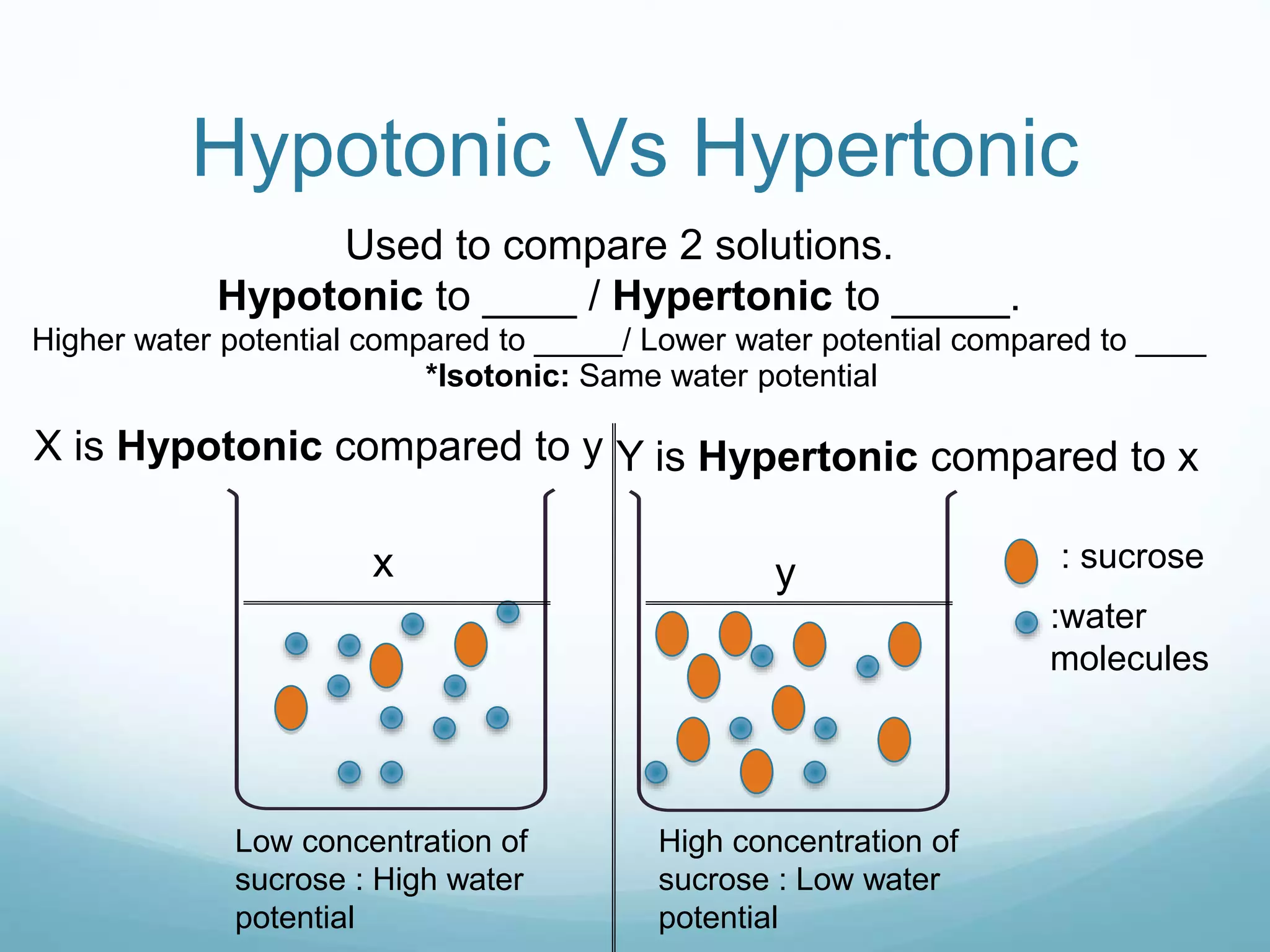
2) Key biological processes like diffusion, osmosis, viscosity and surface tension are governed by fundamental physics laws and concepts. Diffusion is the net movement of particles from an area of higher concentration to lower concentration down a concentration gradient. Osmosis is the movement of water molecules through a partially permeable membrane from a solution of higher water potential to lower water potential.
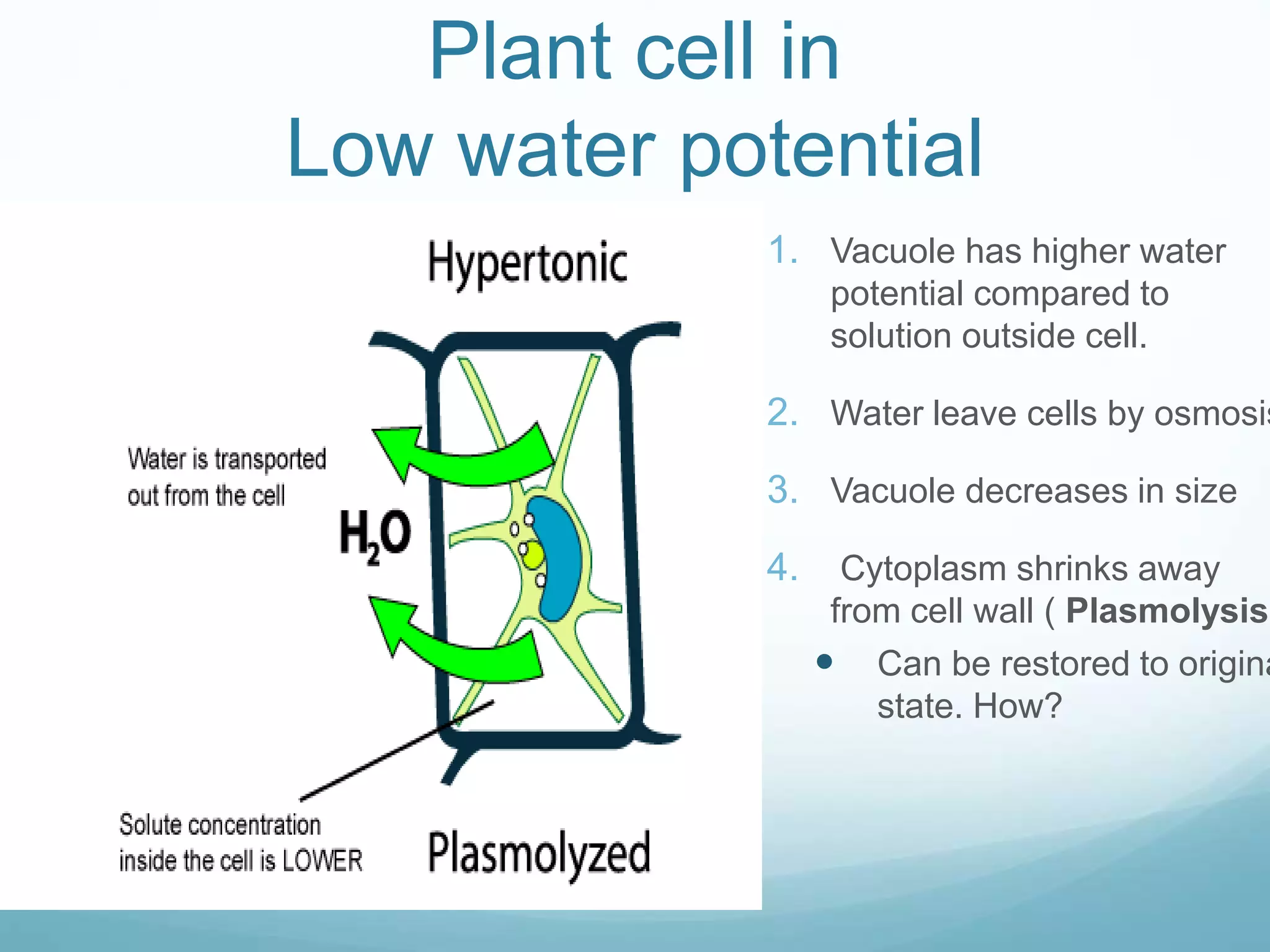
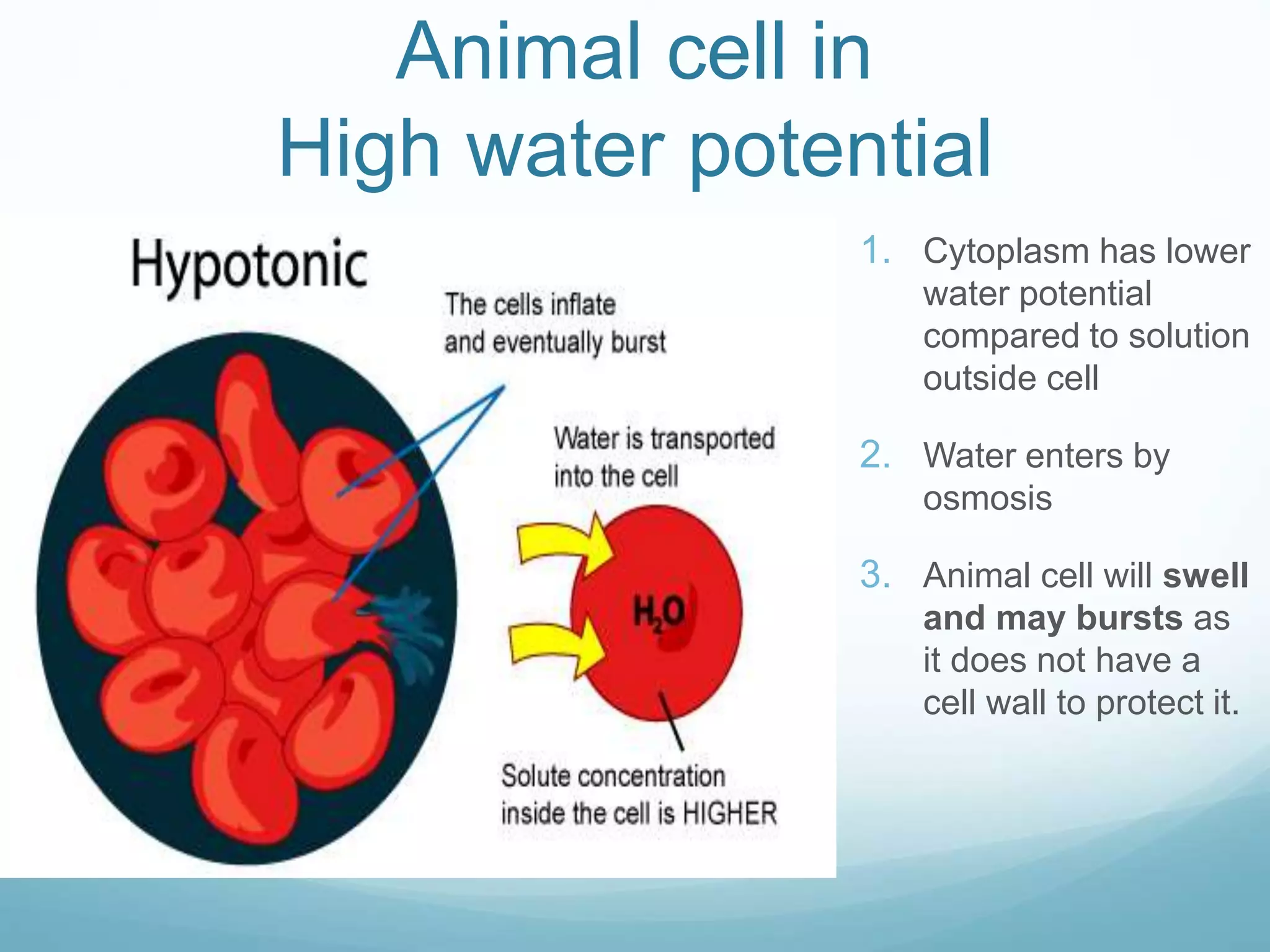
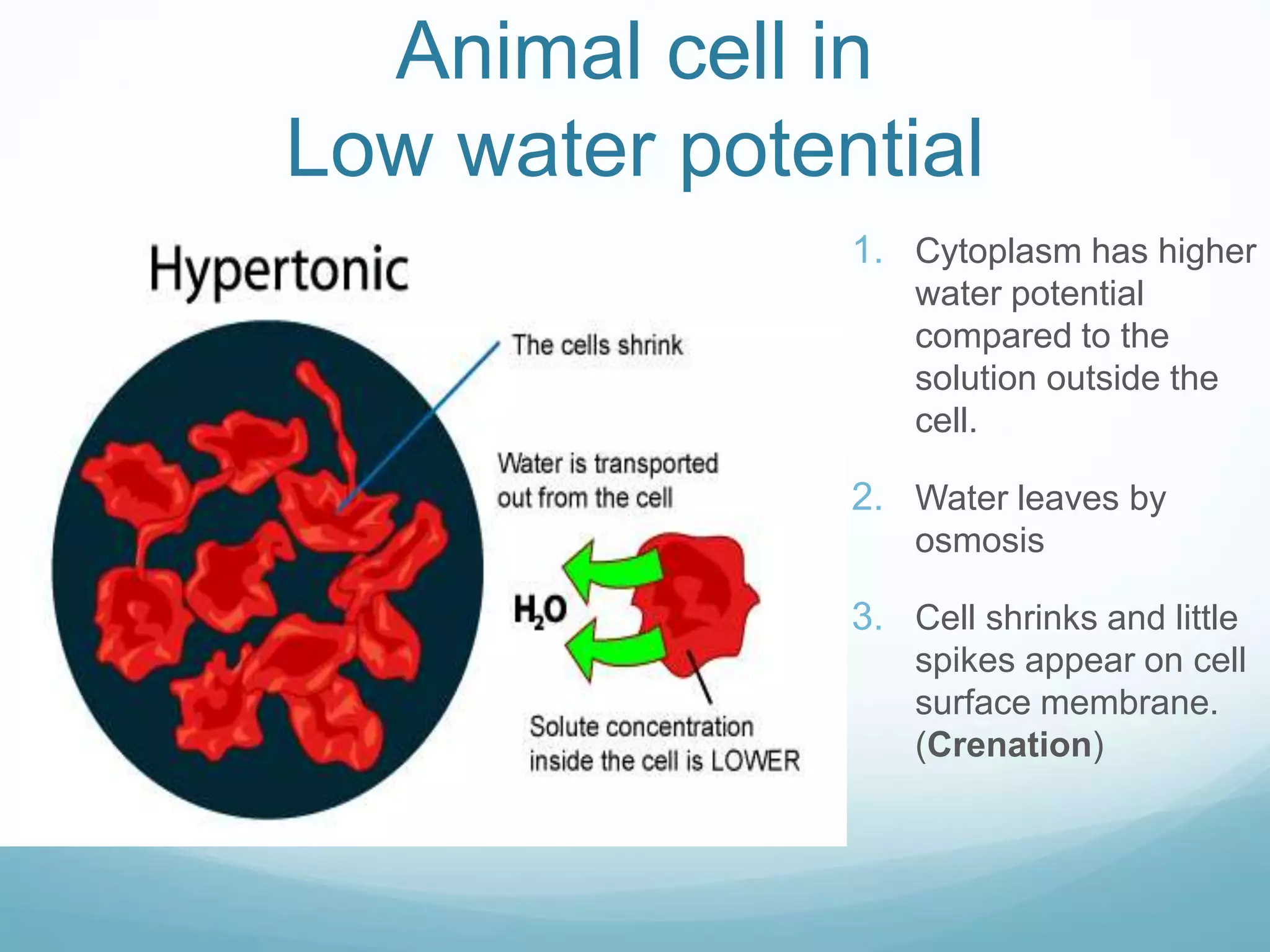
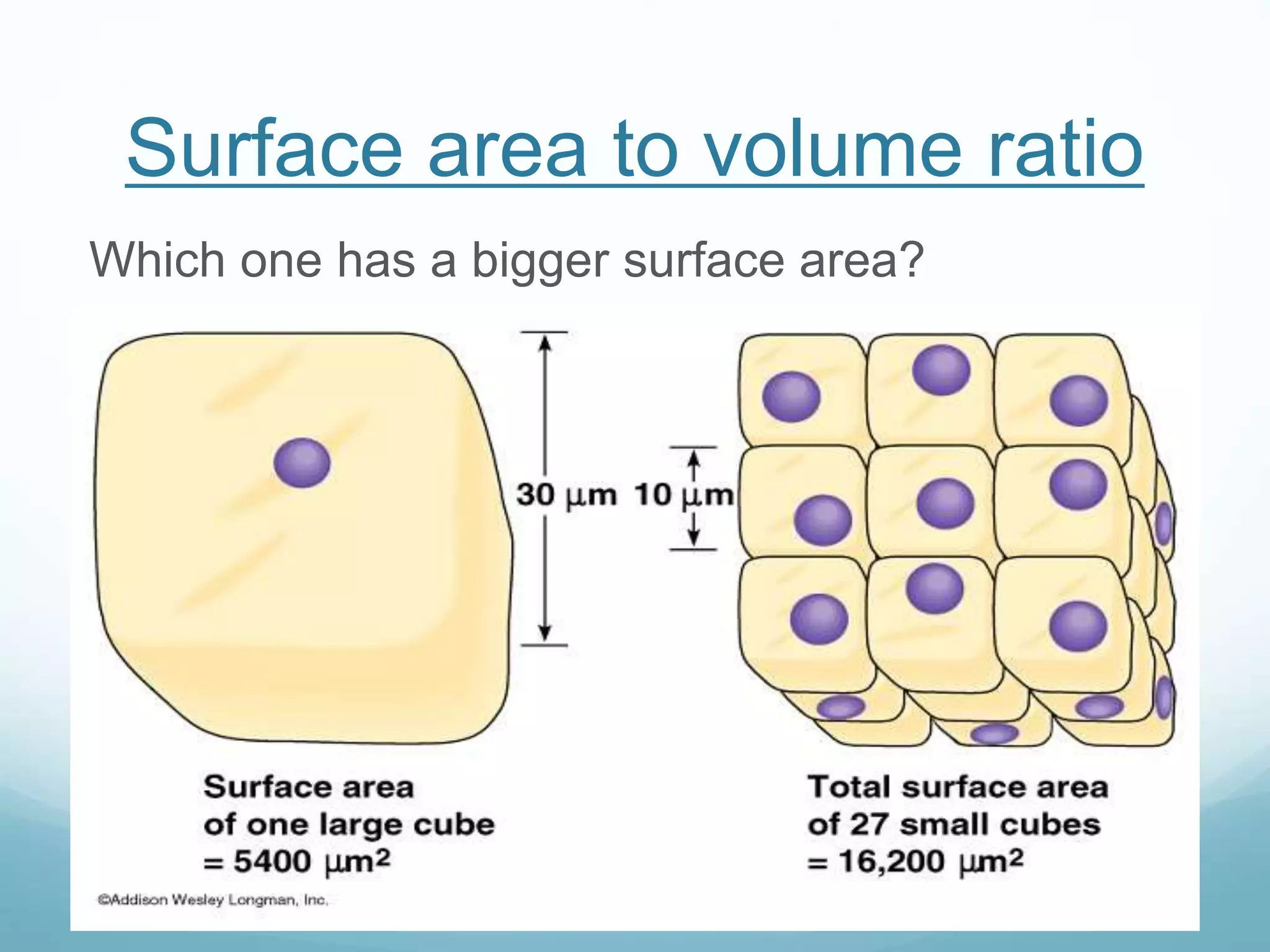
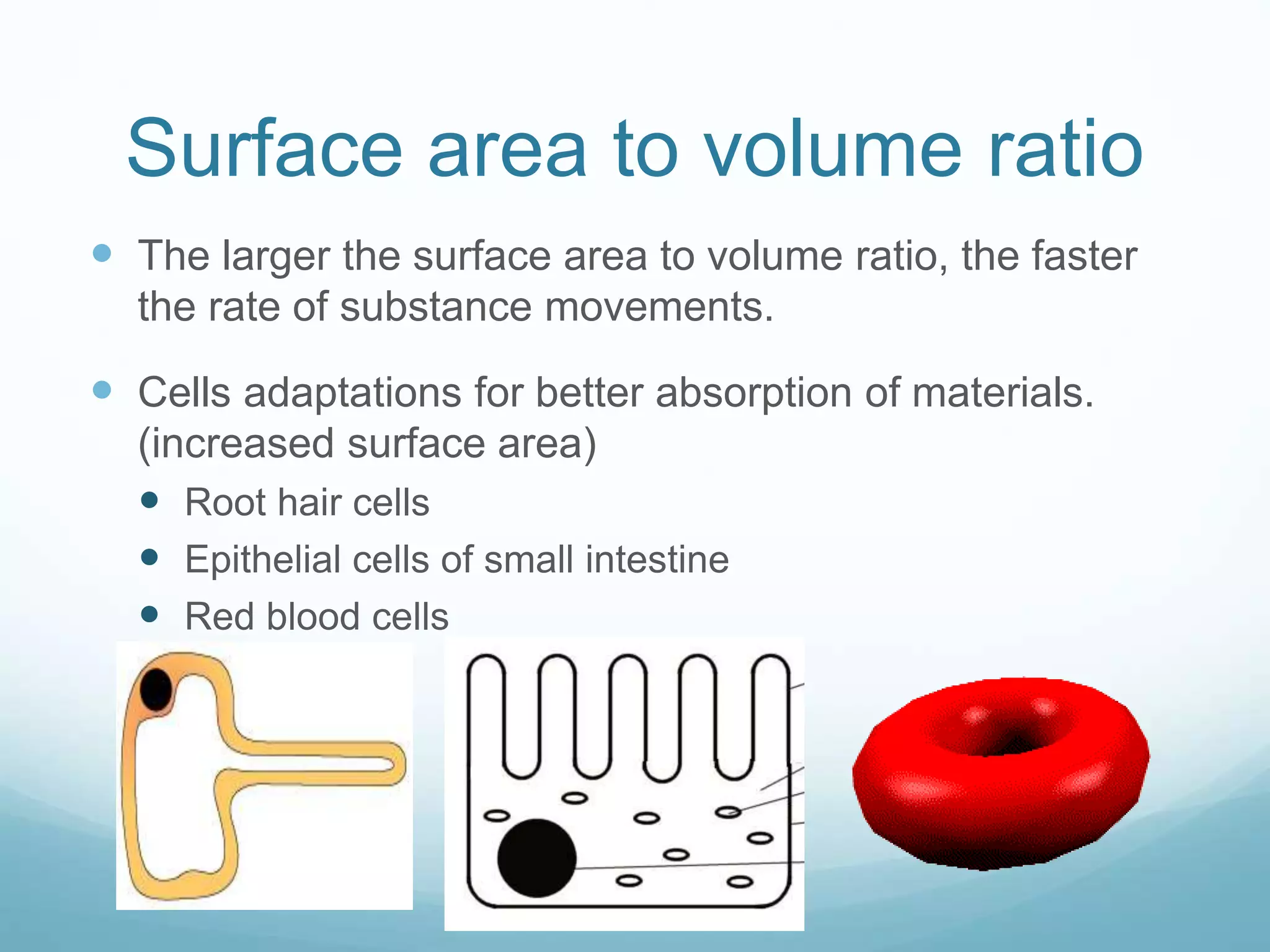
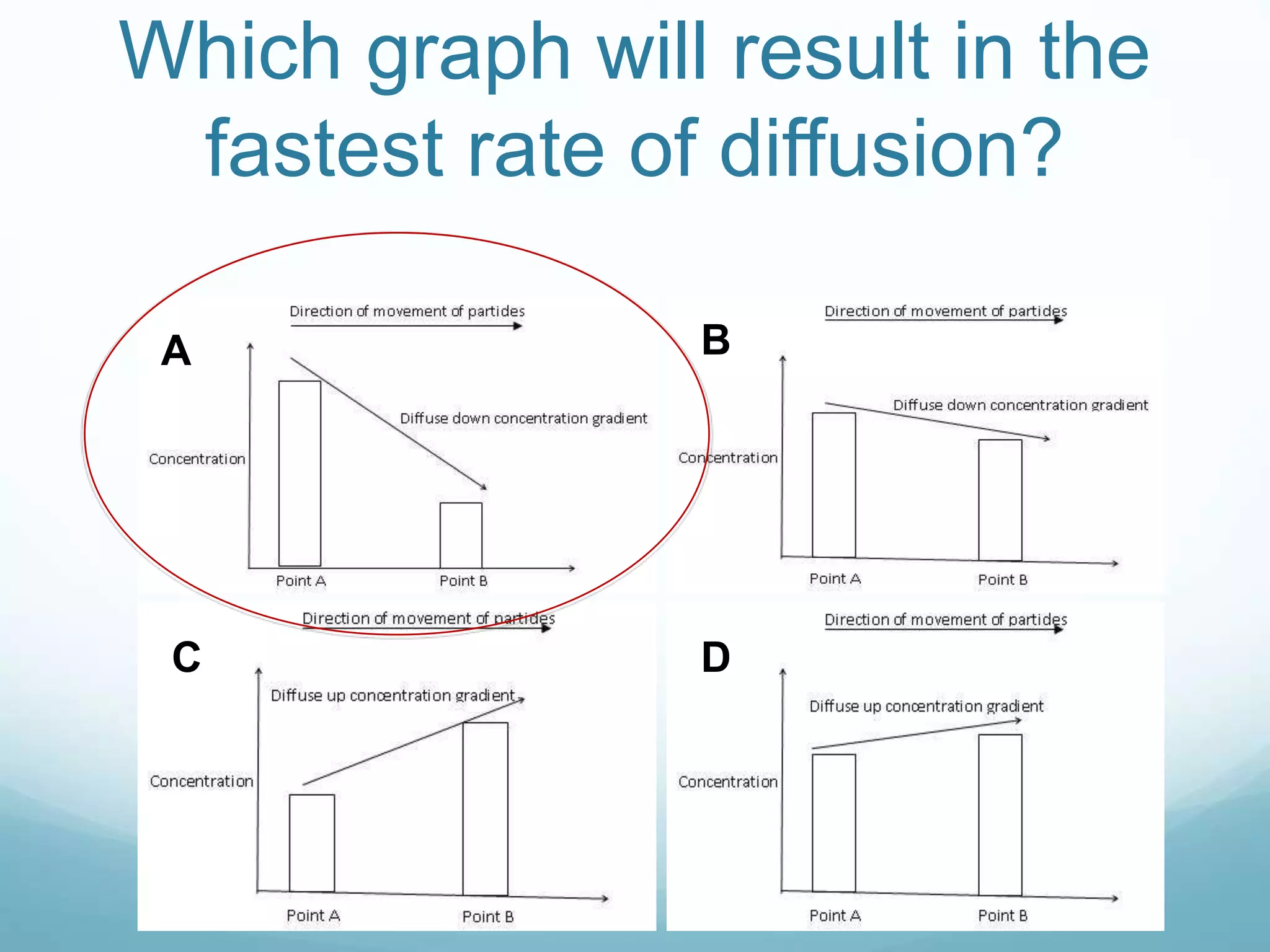
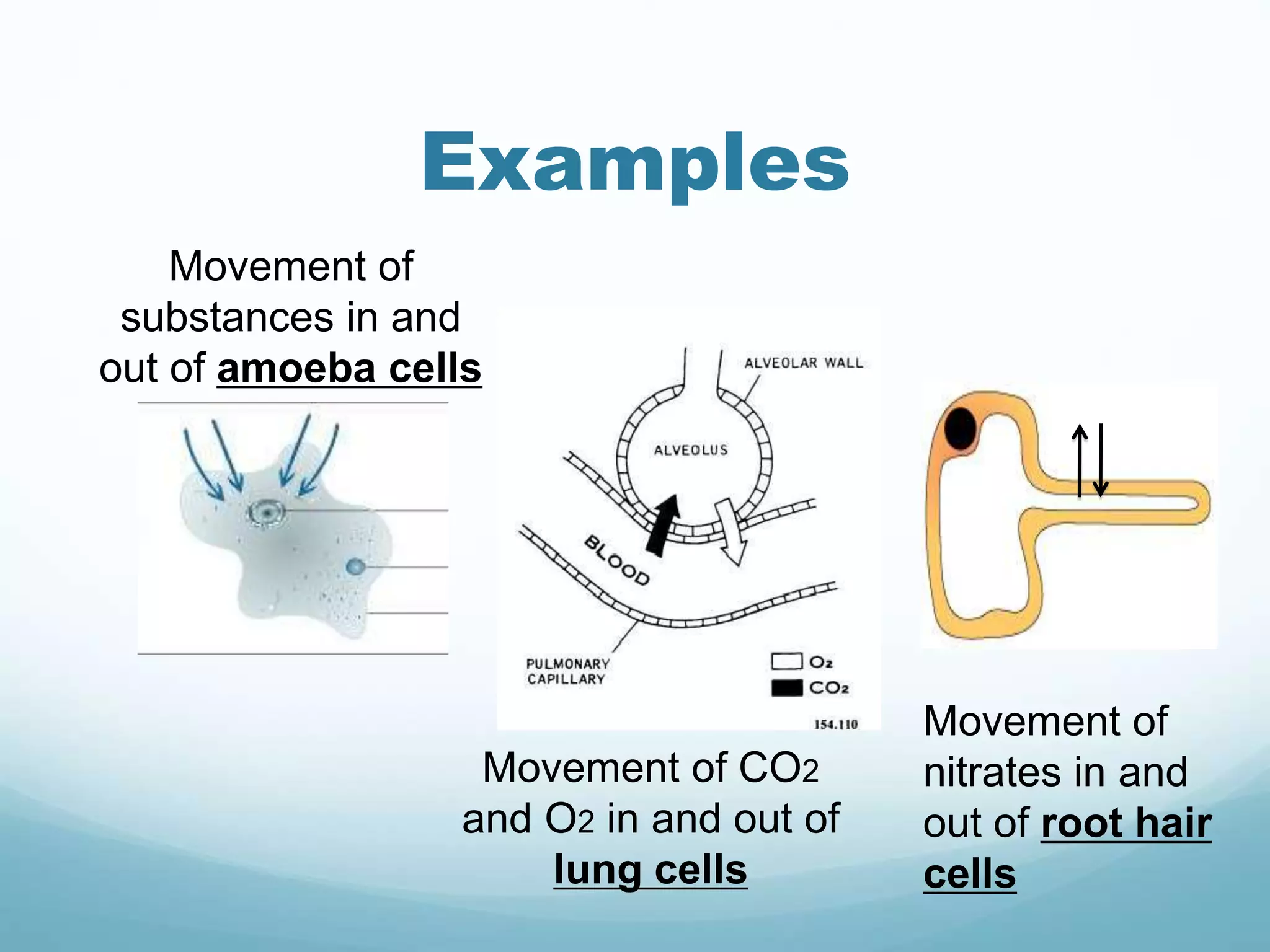
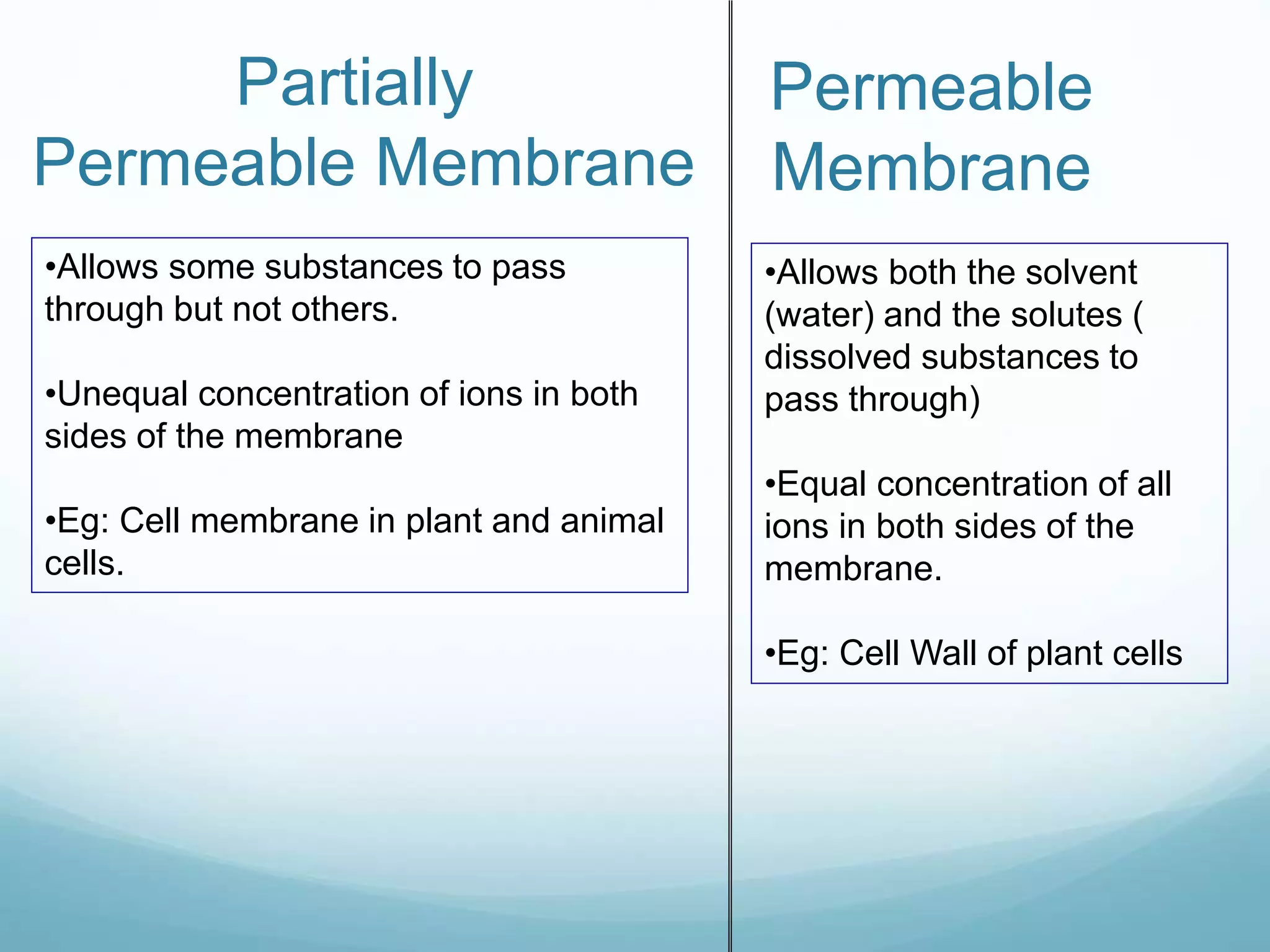
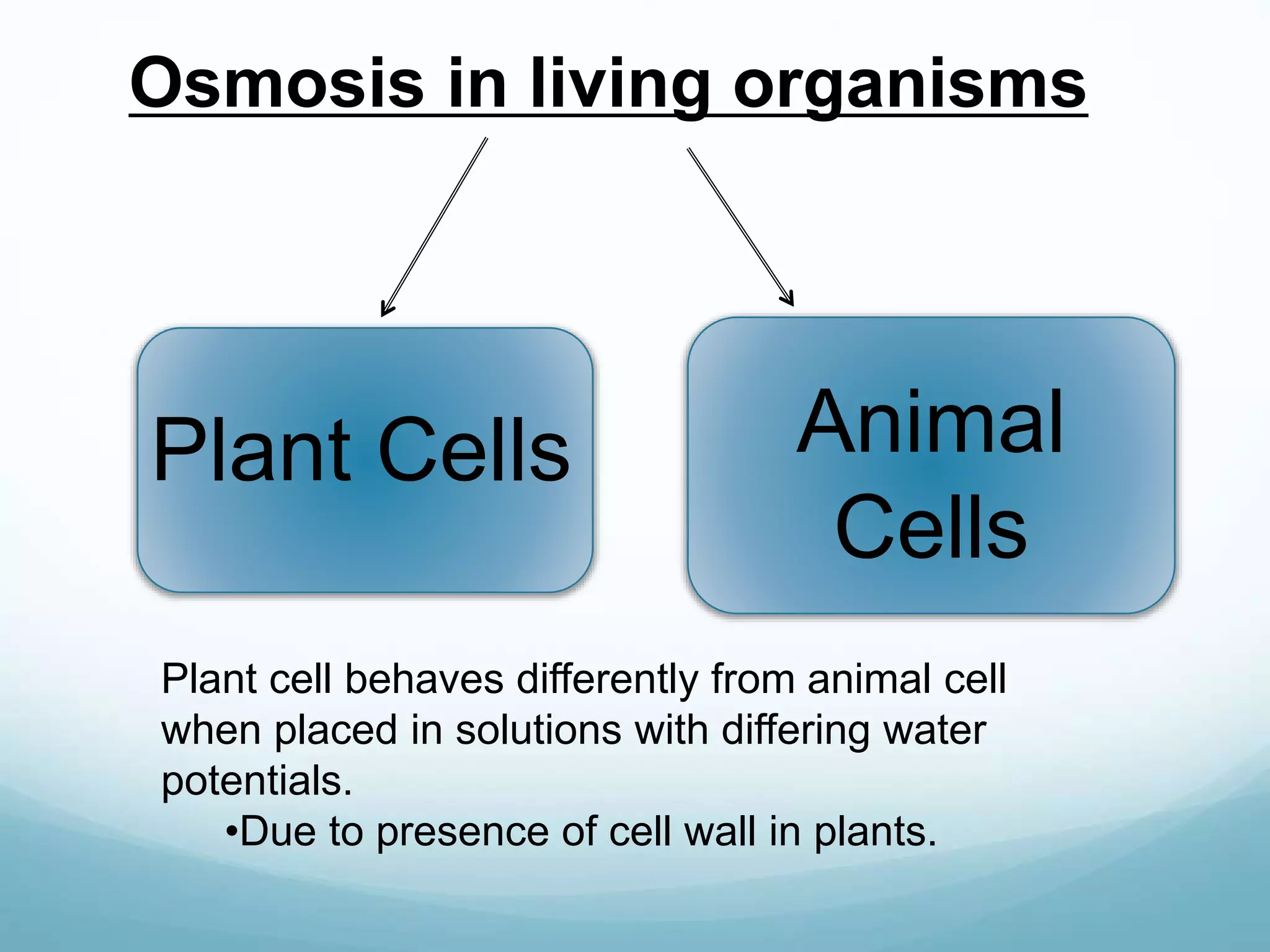
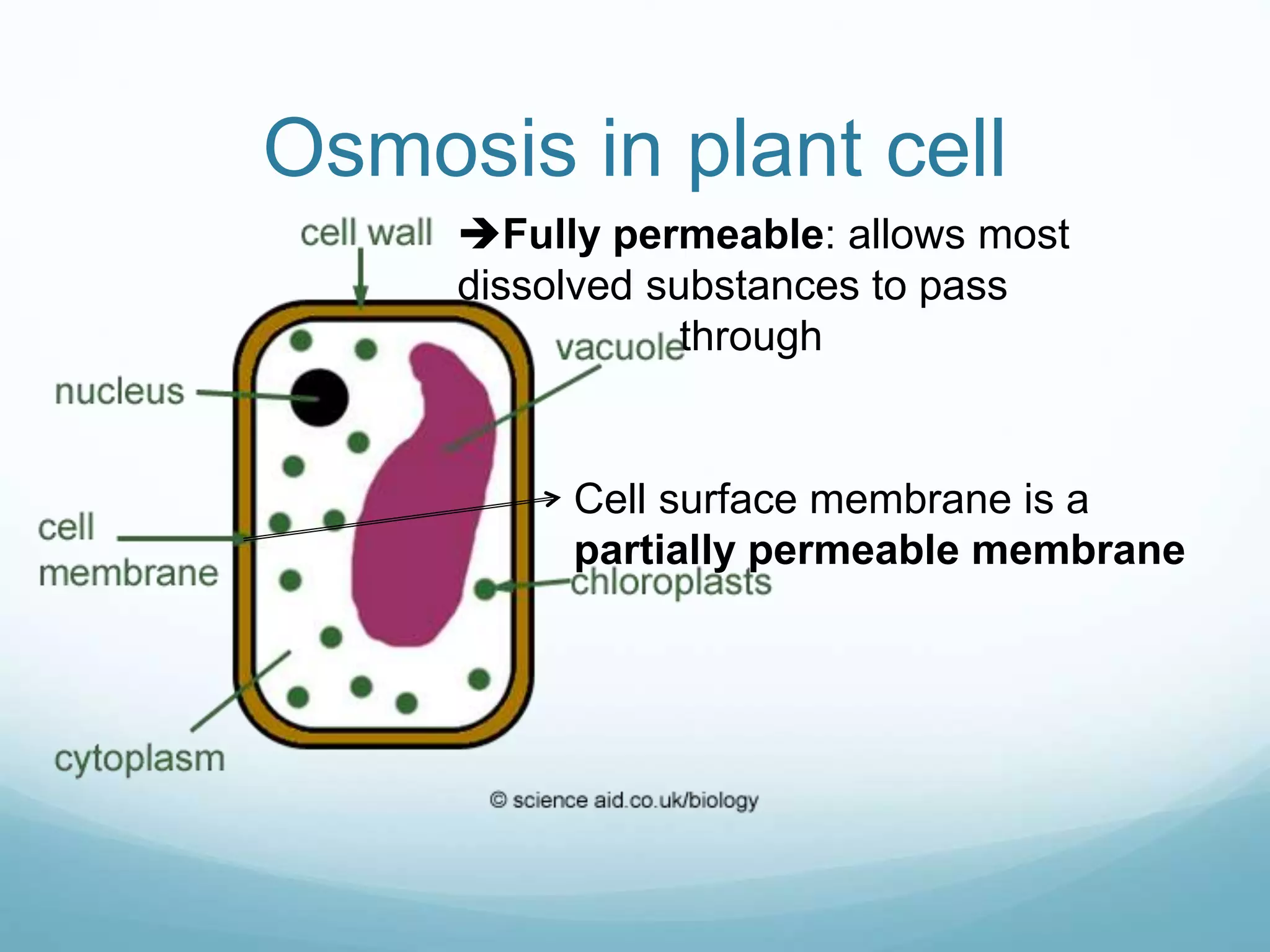
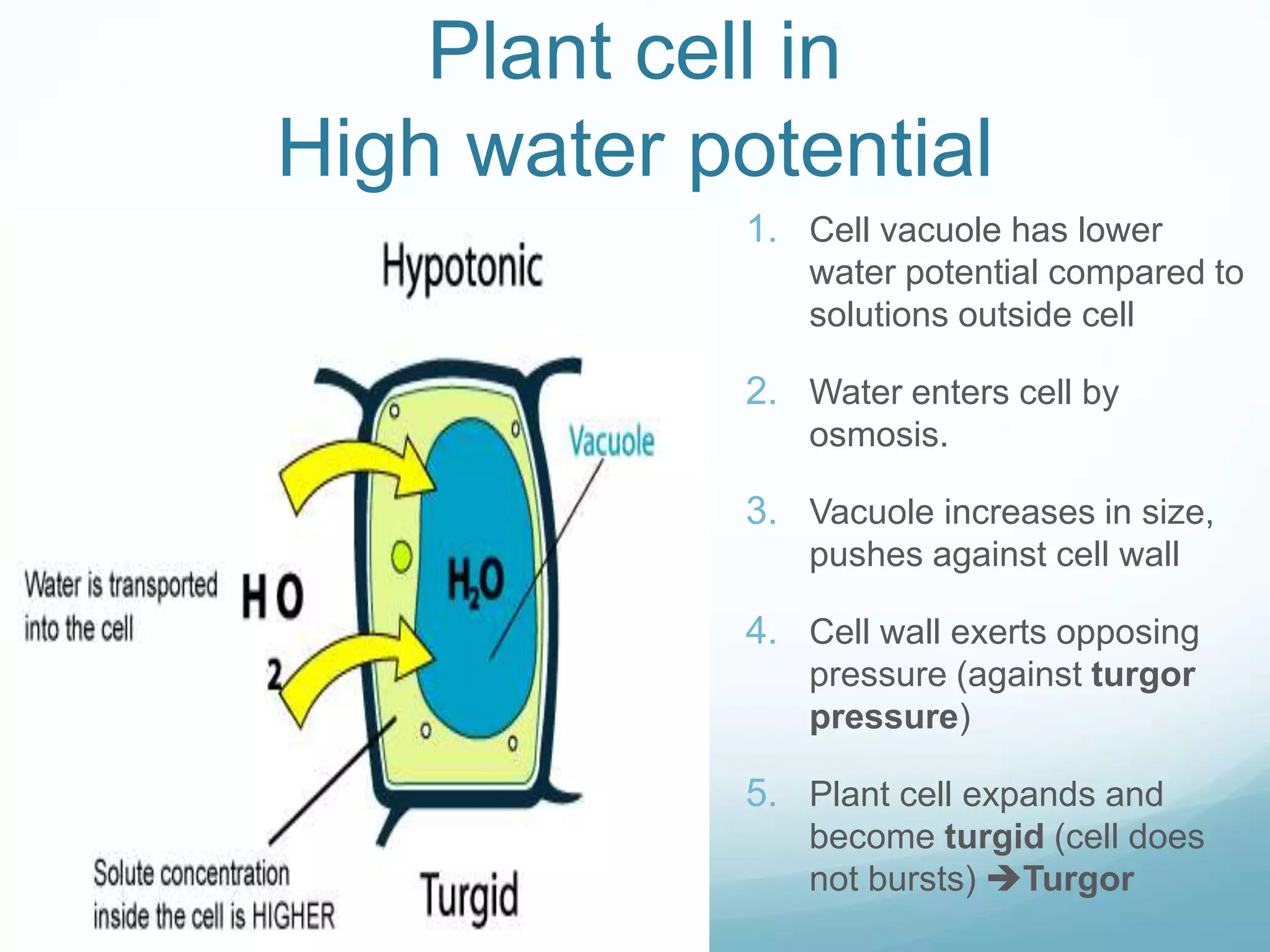
3) Diffusion and osmosis play important roles like facilitating the movement of substances into and out of cells. The behavior of plant and animal cells differs when placed in solutions of varying water potentials due





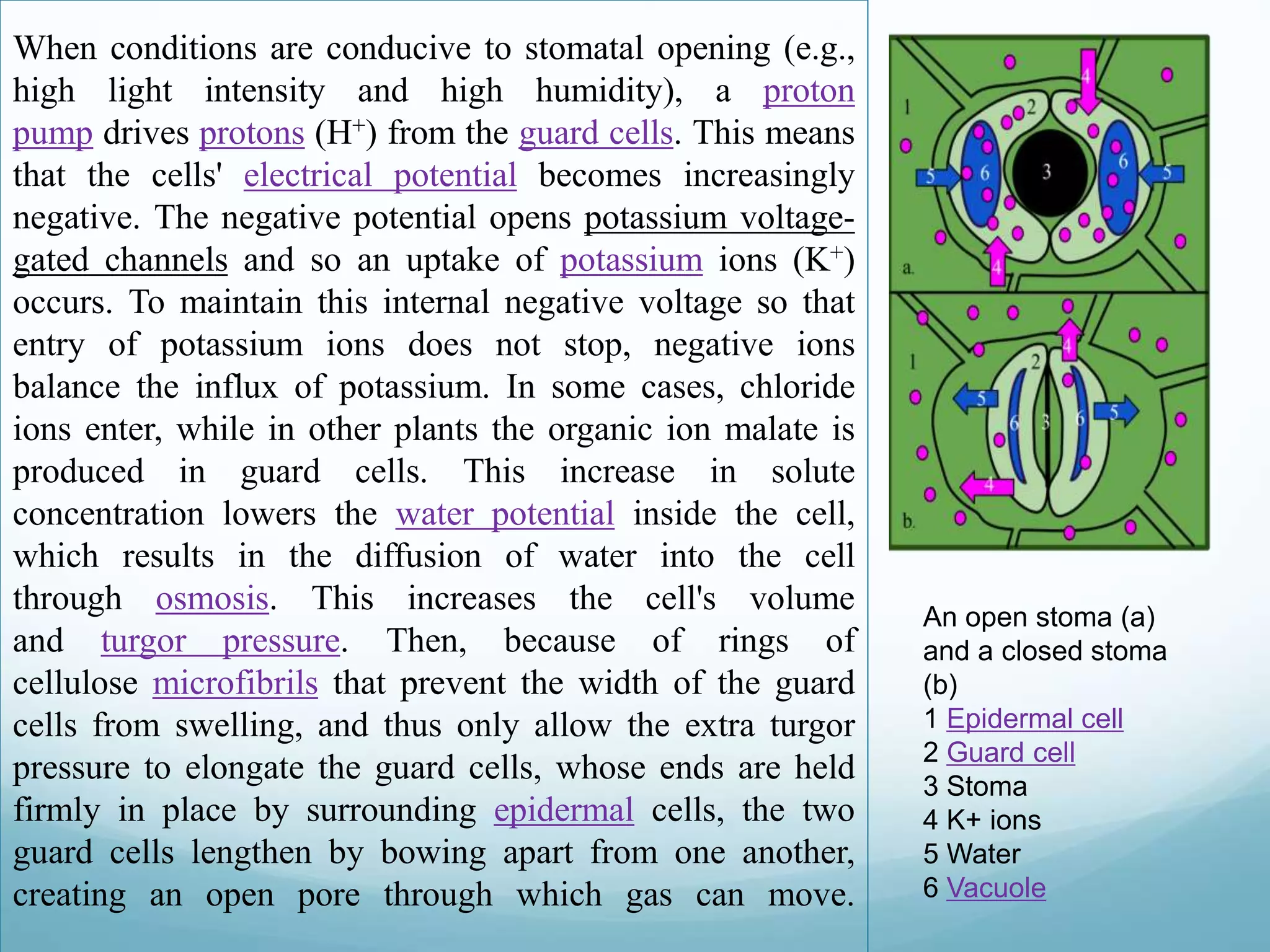
![When the roots begin to sense a water shortage
in the soil, abscisic acid (ABA) is
released.[6] ABA binds to receptor proteins in
the guard cells' plasma membrane and cytosol,
which first raises the pH of the cytosol of the
cells and cause the concentration of free
Ca2+ to increase in the cytosol due to influx
from outside the cell and release of Ca2+ from
internal stores such as the endoplasmic
reticulum and vacuoles.[7] This causes the
chloride (Cl-) and inorganic ions to exit the
cells. Second, this stops the uptake of any
further K+ into the cells and, subsequently, the
loss of K+. The loss of these solutes causes an
increase in water potential, which results in the
diffusion of water back out of the cell
by osmosis. This makes the cel lplasmolysed,
which results in the closing of the stomatal
pores.
An open stoma (a) and a
closed stoma (b)
1 Epidermal cell
2 Guard cell
3 Stoma
4 K+ ions
5 Water
6 Vacuole](https://image.slidesharecdn.com/lect1biochemiical-141118114128-conversion-gate02/75/Lect-1-biochemiical-35-2048.jpg)