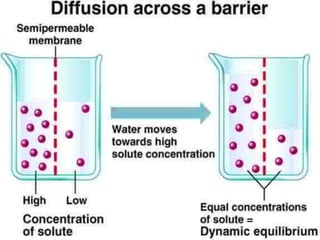
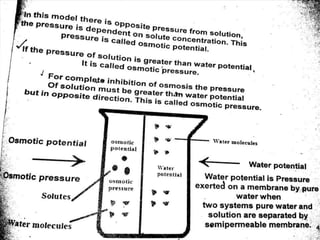
Water potential is a measure of water's ability to do work. It is represented by the Greek letter psi and measured in pascals. Water potential is equal to the sum of solute potential and pressure potential. Solute potential decreases as solute concentration increases, making water potential more negative. Pressure potential increases with pressure, making water potential less negative. Water will always move from areas of higher water potential to lower water potential through semipermeable membranes.