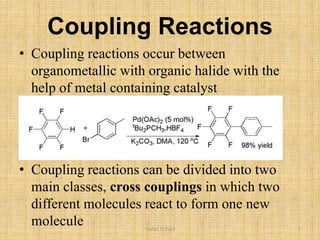
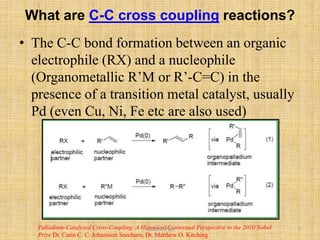
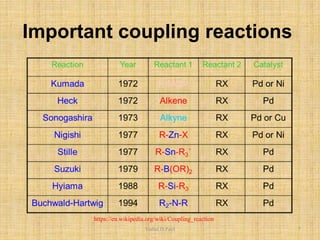
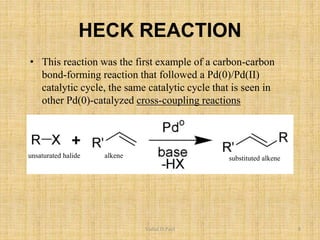
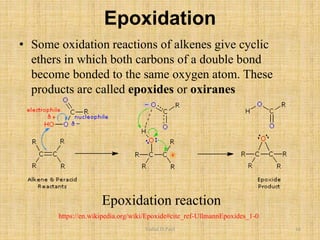
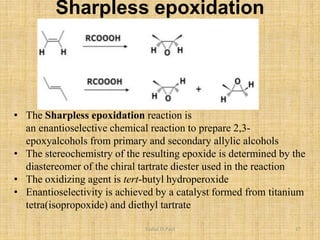
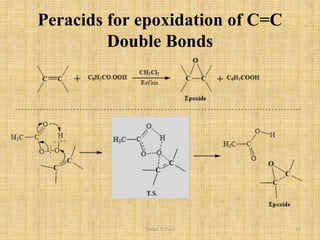
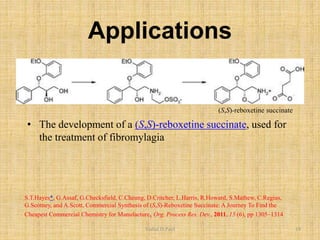
i. The document presents information on three important coupling reactions: the Heck reaction, Suzuki coupling, and Sharpless epoxidation.
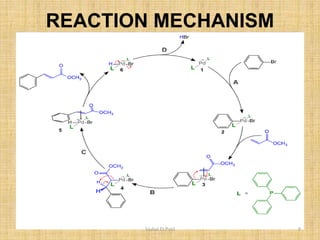
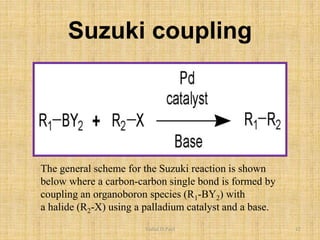
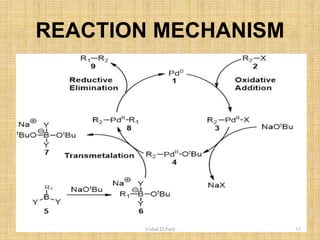
ii. The Heck reaction forms carbon-carbon bonds between an alkene and an unsaturated halide. The Suzuki coupling couples an organoboron compound with a halide using palladium catalysis. The Sharpless epoxidation reaction produces chiral epoxides from allylic alcohols in high enantiomeric excess.
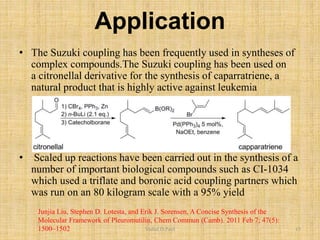
iii. Examples of applications are given for drug synthesis using these reactions.