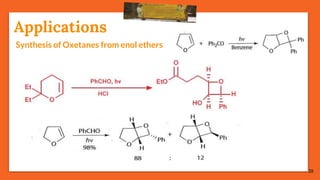
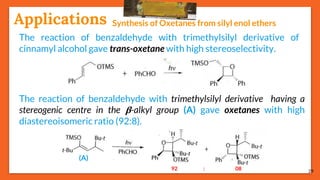
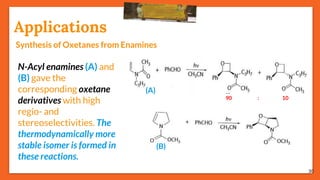
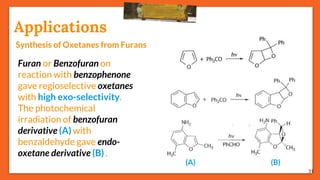
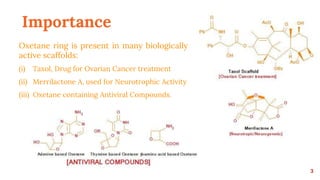
The Paternò-Büchi reaction involves the photochemical reaction between a carbonyl compound and an alkene to form an oxetane ring. This reaction was first reported in 1909 by Paternò and Chieffi. Several mechanisms are possible, including those involving a diradical intermediate or photoinduced electron transfer. The reaction shows regioselectivity, site selectivity, and stereoselectivity that depend on factors such as the solvent, substituents on the carbonyl compound or alkene, and temperature. The Paternò-Büchi reaction has been used to synthesize various natural products and allows formation of oxetane rings, which are present in several biologically active compounds.


![Mechanism
[Mechanisms involving a diradical intermediate]
5
❏ The carbonyl compound is excited to a
singlet state (usually n*) and then reaches a
triplet state after an ISC
❏ The diradical intermediate 1D,formed by
attack of the singlet excited carbonyl
compound on the alkene, mainly leads to its
triplet counterpart 3D.
❏ After a second ISC,which is controlled by a
spin-orbit coupling, this diradical 3D can
undergo either cyclization to the oxetane
or cleavage of the new C-O covalent bond
to regenerate the substrates in their
ground state.
one or several diradical
intermediates are involved](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-5-320.jpg)

![Mechanism
The semi-occupied np orbital of the oxygen atom is electrophilic,
whereas the single electron of the π* orbital induces a nucleophilic
character of the system of the carbonyl compound (3-electron
system)
7
[Influence of the amphoteric character of the carbonyl compound at
its nπ* state]](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-7-320.jpg)
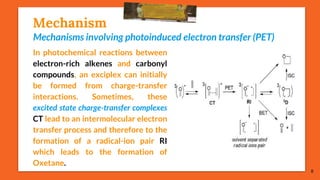
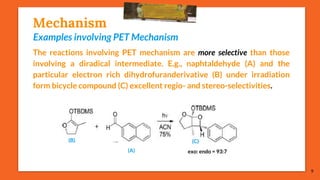
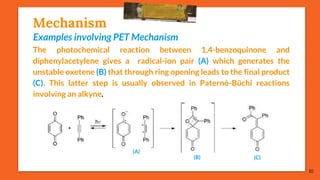
![Mechanism
[Paternò-Büchi reaction in the ππ* state of the carbonyl compound]
The carbonyl compounds which possess a ππ* state lower in energy than
their nπ* state undergo a Paternò-Büchi reaction with higher quantum yields
than those which react in their nπ*state. E.g., photochemical excited acetyl-
selenophene (A) is more stable in its ππ* triplet state. The oxetane (C) is thus
the result of a nucleophilic attack of the π system of the carbonyl
compound on tetramethylethylene (B). The competitive product (D)
results from the [2 + 2] cycloaddition of the alkene to the heterocycle.
11
(A) (B)
(C) (D)](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-11-320.jpg)
![Mechanism
[Solvent Effect]
12
The solvent determines the nature of the mechanism involved. E.g., if the reaction
between benzaldehyde and 2,3-dihydrofuran is performed in
High regioselectivity
Non-polar solvents (benzene) Diradical intermediate
Mechanism
Low regioselectivity
Polar solvents (Acetonitrile) PET Mechanism
Solvent (A) : (B)
Benzene 29 : 01
Acetonitrile 05 : 01
(A) (B)](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-12-320.jpg)
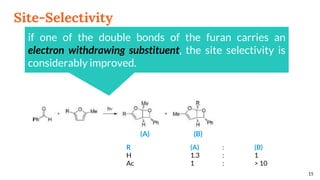
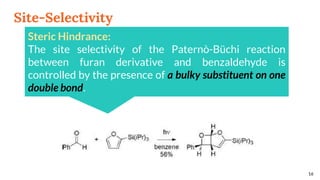
![Selectivity
Site selectivity
describes the preferred
attack of the carbonyl
compound on a
particular double bond,
when the alkene
partner possesses two
or more double bonds
Regioselectivity, which
corresponds, as for all [2 + 2]
cycloadditions, to the “head-to-
head” or “head-to-tail”
connectivity of both reagents.
13
Site Selectivity Regioselectivity](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-13-320.jpg)
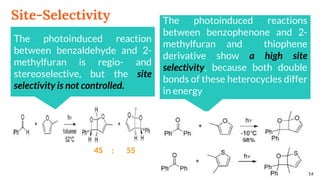

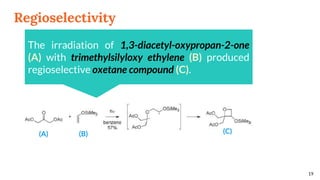
![Regioselectivity
18
In the reaction between benzophenone and the uracil
derivatives, the structure of the main regioisomer
depends directly on the position of the methyl on the
double bond
R R’ (A) : (B) Yield [%]
Me H 71 : 29 51
H Me 19 : 81 44
(A) (B)](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-18-320.jpg)
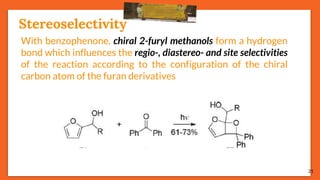
![Stereoselectivity
20
In the photoinduced reaction
between furan and several
aromatic carbonyl compounds, the
exo/endo stereoselectivity depends
directly on the substituent of the
carbonyl compound
R (exo) : (endo)
H 212 : 1
Me >49 : 1
CN 3.7 : 1
CO2Me 1 : 9
(exo) (endo)
R (A) : (B)
H 89 : 11
Me 50 : 50
(A) (B)
When R = H, irradiation
of compounds [X] and
[Y] selectively yields
oxetane (A), but no
selectivity is observed
when R = Me
(X)
(Y)](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-20-320.jpg)
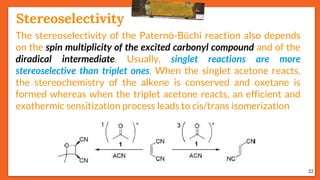
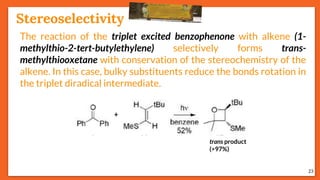
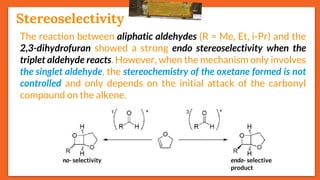
![Stereoselectivity
25
The temperature has an influence on the selectivity of the Paternò-
Büchi reaction when the mechanism involves a diradical
intermediate and when one of the possible diradical is
thermodynamically favored. E.g., stereoselectivity of the
photoinduced reaction between benzophenone and cyclooctene
depends on temperature.
trans
Stereoselectivity
Effect of Temperature on Selectivity
ciscis / trans
Octene Temp.[°C] (trans) : (cis)
cis - 95 02 : 98
cis + 110 80 : 20
trans - 80 96 : 04
Trans + 110 90 : 10](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-25-320.jpg)
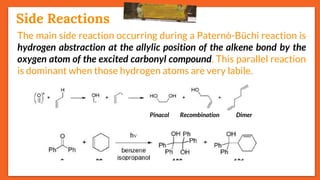
![Stereoselectivity
27
Another side reaction of the Paternò-Büchi reaction is the Norrish
type-II reaction (𝜸-hydrogen atom abstraction). E.g., 𝝰-ketoester
[Ethyl(phenyl)glyoxylate] on reaction with electron-rich alkenes
such as cyclohexa-1,3-diene yields oxetane under irradiation.
However, with electron-poor alkenes such as allyl bromide,
ethyl(phenyl)glyoxylate is fragmented according to a Norrish type-II
reaction via the typical intermediate.
Side Reactions](https://image.slidesharecdn.com/paterno-buchireaction-200522152647/85/Paterno-buchi-reaction-27-320.jpg)