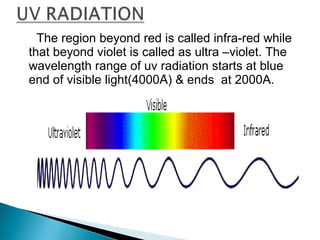
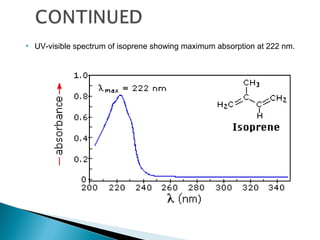
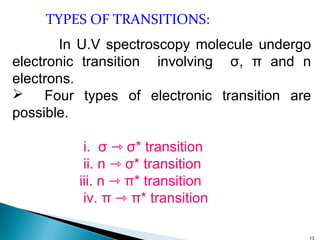
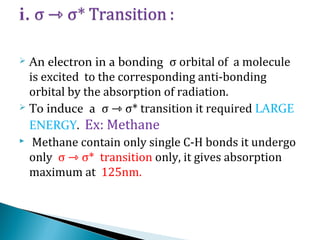
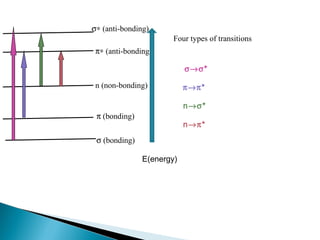
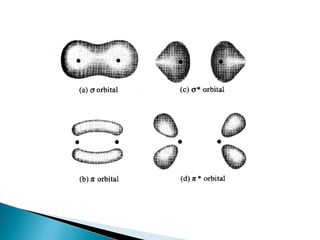
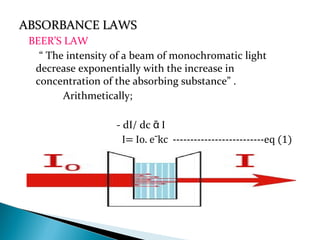
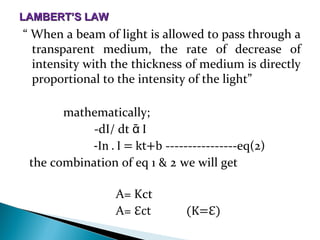
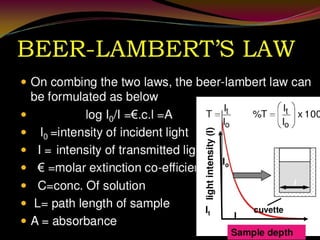
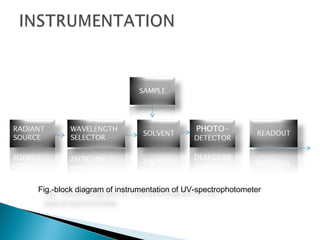
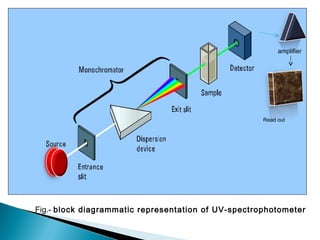
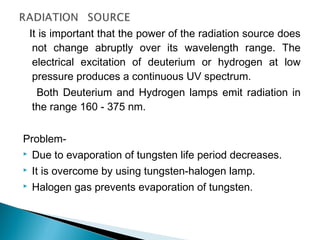
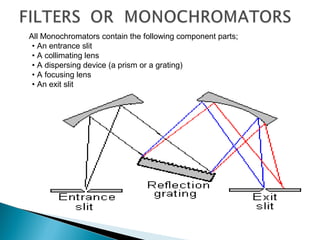
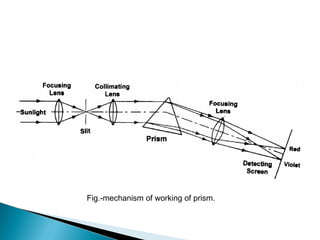
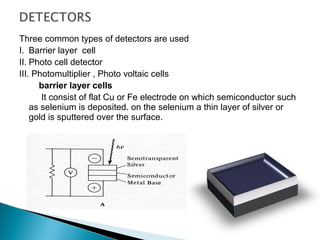
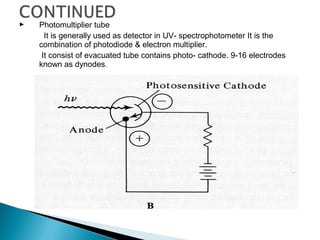
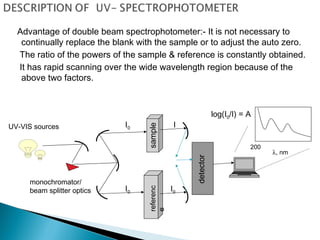
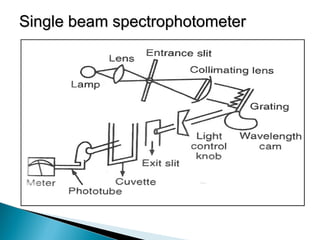
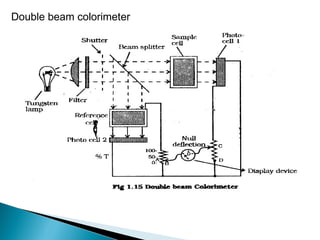
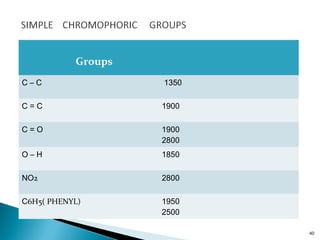
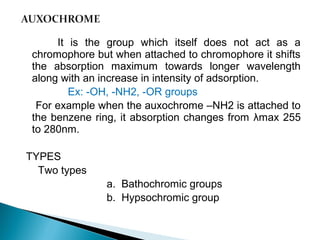
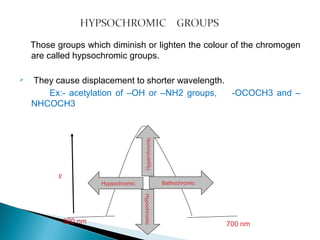
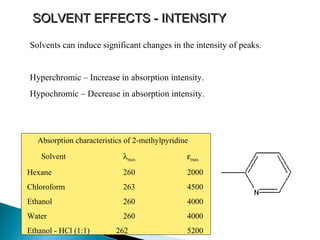
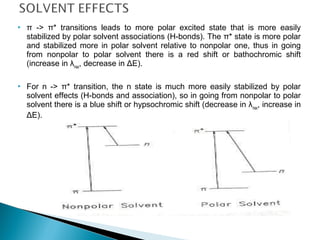
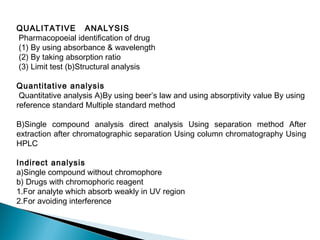
This document provides an overview of ultraviolet-visible (UV-Vis) spectroscopy. It defines UV-Vis spectroscopy as the measurement of light absorption by a sample after it passes through or is reflected from the sample. The document outlines key components of UV-Vis spectroscopy including the absorption spectrum, types of electronic transitions that can occur, Beer's and Lambert's laws describing the relationship between absorbance and concentration, instrumentation components, and applications such as qualitative and quantitative analysis. Effects of chromophores, solvents, and auxochromes on absorption spectra are also discussed.