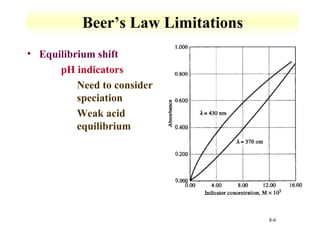
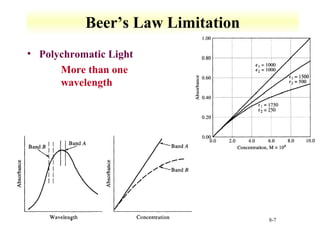
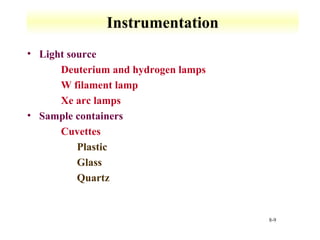
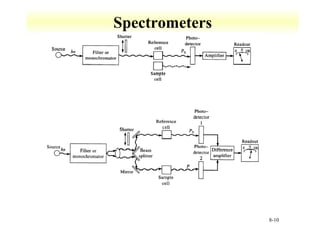
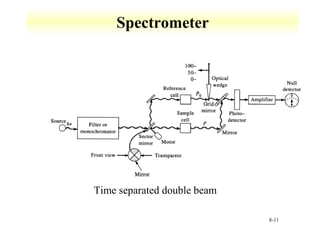
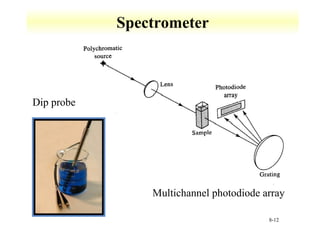
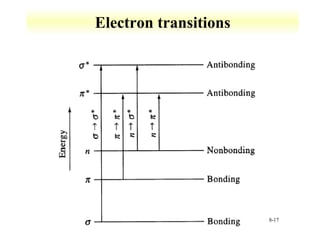
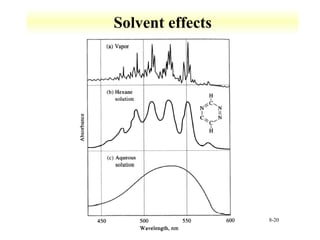
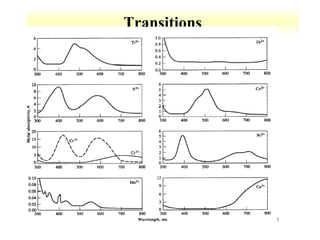
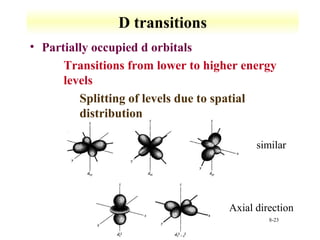
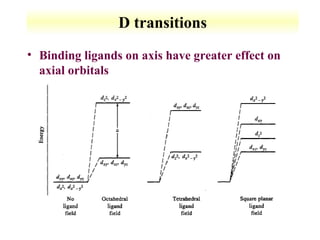
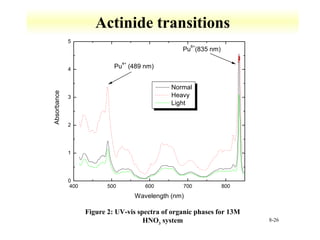
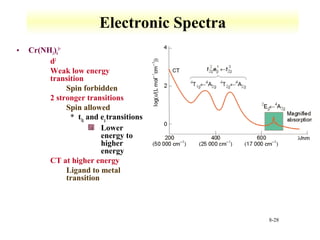
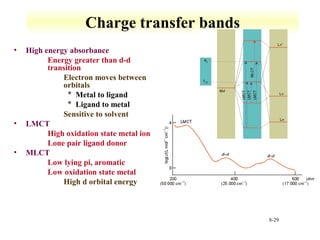
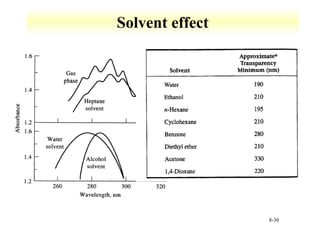
UV-Vis spectroscopy measures the absorption of ultraviolet and visible light by molecules. It can detect electronic transitions in molecules, which follow Beer's law - absorbance is proportional to concentration. UV-Vis is used to identify organic and inorganic compounds and determine concentrations. Instrumentation includes light sources, sample containers, and spectrometers to separate wavelengths and detect absorbances. Applications include titrations and measuring charge-transfer transitions between electron donors and acceptors.