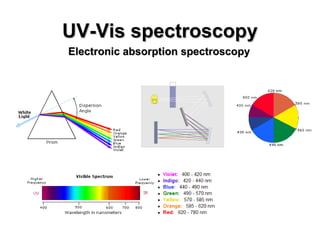
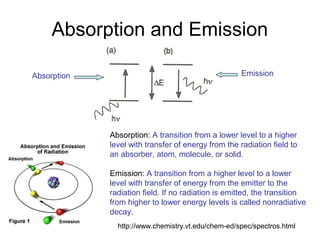
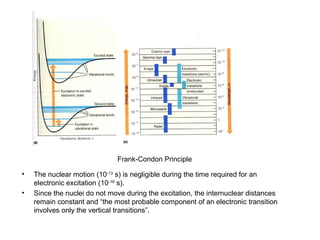
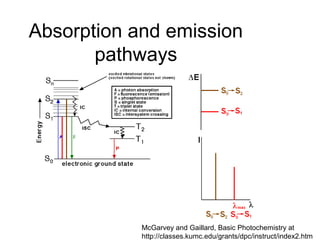
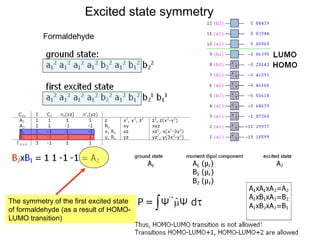
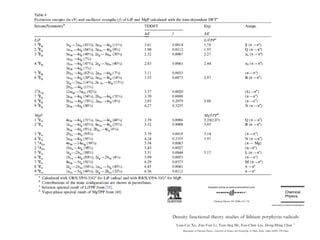
This document summarizes UV-Vis spectroscopy. It describes how UV-Vis spectroscopy can provide information about unsaturated functional groups and concentration. It also discusses absorption and emission, the Frank-Condon principle, selection rules for electronic transitions, and calculation of electronic spectra using time-dependent density functional theory.