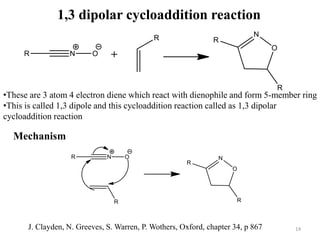
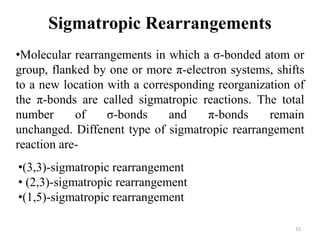
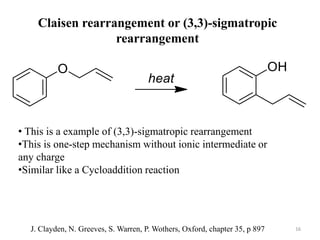
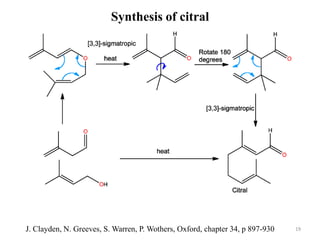
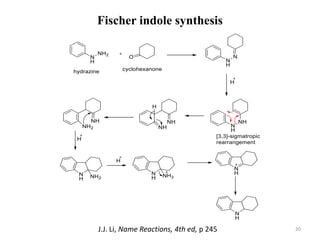
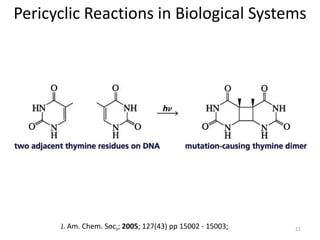
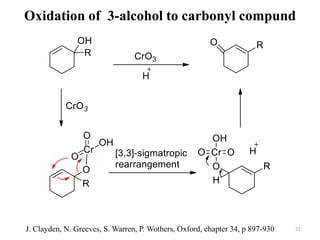
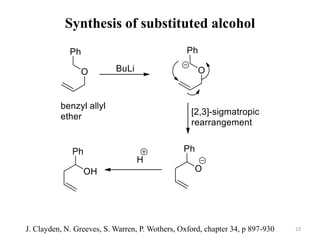
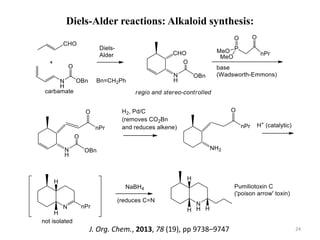
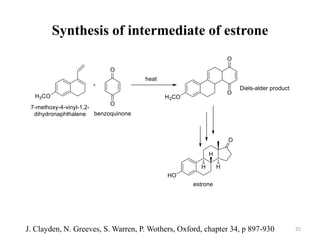
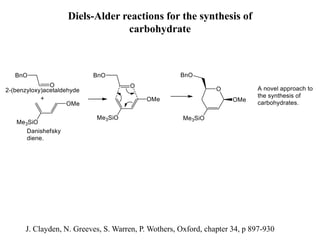
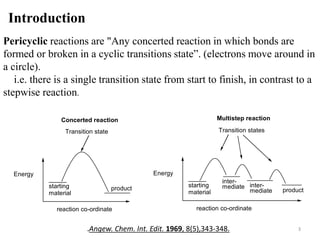
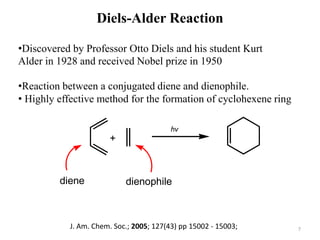
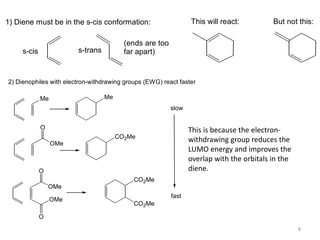
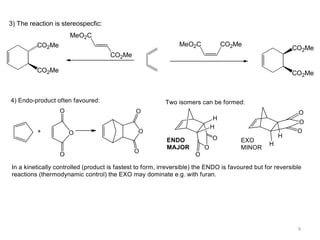
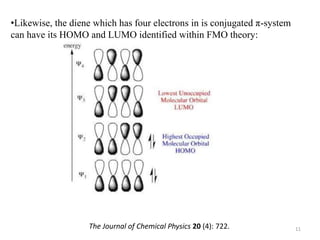
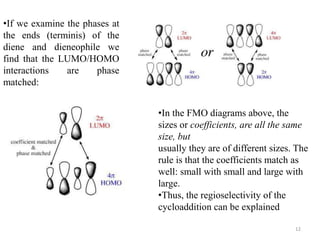
Pericyclic reactions involve the formation or breaking of bonds in a cyclic transition state. They include cycloadditions, electrocyclic reactions, sigmatropic rearrangements, and others. Cycloadditions like the Diels-Alder reaction involve the combination of unsaturated molecules to form a cyclic adduct. The Diels-Alder reaction between a conjugated diene and dienophile forms a cyclohexene ring. Frontier molecular orbital theory can explain the regioselectivity of cycloadditions. Examples of pericyclic reactions include the synthesis of citral via a Claisen rearrangement, Fischer indole synthesis, and Diels-Alder reactions in alkaloid and carbohydrate synthesis.



![Cycloaddition reaction
• A cycloaddition is a pericyclic chemical reaction, in which
"two or more unsaturated molecules (or parts of the same
molecule) combine with the formation of a cyclic adduct in
which there is a net reduction of the bond multiplicity." The
resulting reaction is a cyclization reaction. Designated as
[A+B].
• A and B refers to number of atoms containing π-electrons
• Three important classes of cycloaddition reactions
(i) Diels-Alder reaction
(iii) [2+2] Cycloaddition
(ii) [1,3]-Dipolar cycloaddition
6](https://image.slidesharecdn.com/pericyclicreactionsppt-200522123929/85/Pericyclic-reactions-6-320.jpg)

![[2+2] Cycloaddition reaction
The [2+2] photocycloaddition is a cycloaddition-type reaction –
it generally entails the formation of new molecules by the reaction
of two unsaturated molecules via two atoms from each molecules
(hence "[2 + 2]").
13Polym. Int. 58 (7): 720](https://image.slidesharecdn.com/pericyclicreactionsppt-200522123929/85/Pericyclic-reactions-13-320.jpg)