The document discusses the nitrogen rule and McLafferty rearrangement. The nitrogen rule states that molecules with an even number of nitrogen atoms have an even nominal mass, while molecules with an odd number of nitrogen atoms have an odd nominal mass. The McLafferty rearrangement is a reaction observed in mass spectrometry where a molecule containing a keto-group undergoes β-cleavage, transferring a hydrogen from the γ position to the carbonyl group. This reaction results in an enol radical cation and a neutral alkene fragment. Any carbonyl compound containing a hydrogen in the γ position is likely to undergo the McLafferty rearrangement during mass spectrometry.







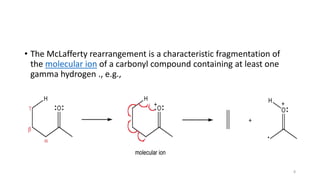



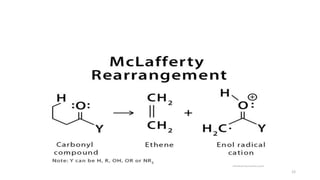

![• Any carbonyl compound that has hydrogen in the gamma
position is likely to have a peak in the mass spectrum
corresponding to the enol radical cation formed by the
McLafferty rearrangement.
• Both ketones and aldehydes give prominent molecular ion
peaks though the [M+] peak is more prominent in ketones.
14](https://image.slidesharecdn.com/mclafferteyrearrangement-221108061307-ebcc2dbb/85/McLaffertey-rearrangement-14-320.jpg)
