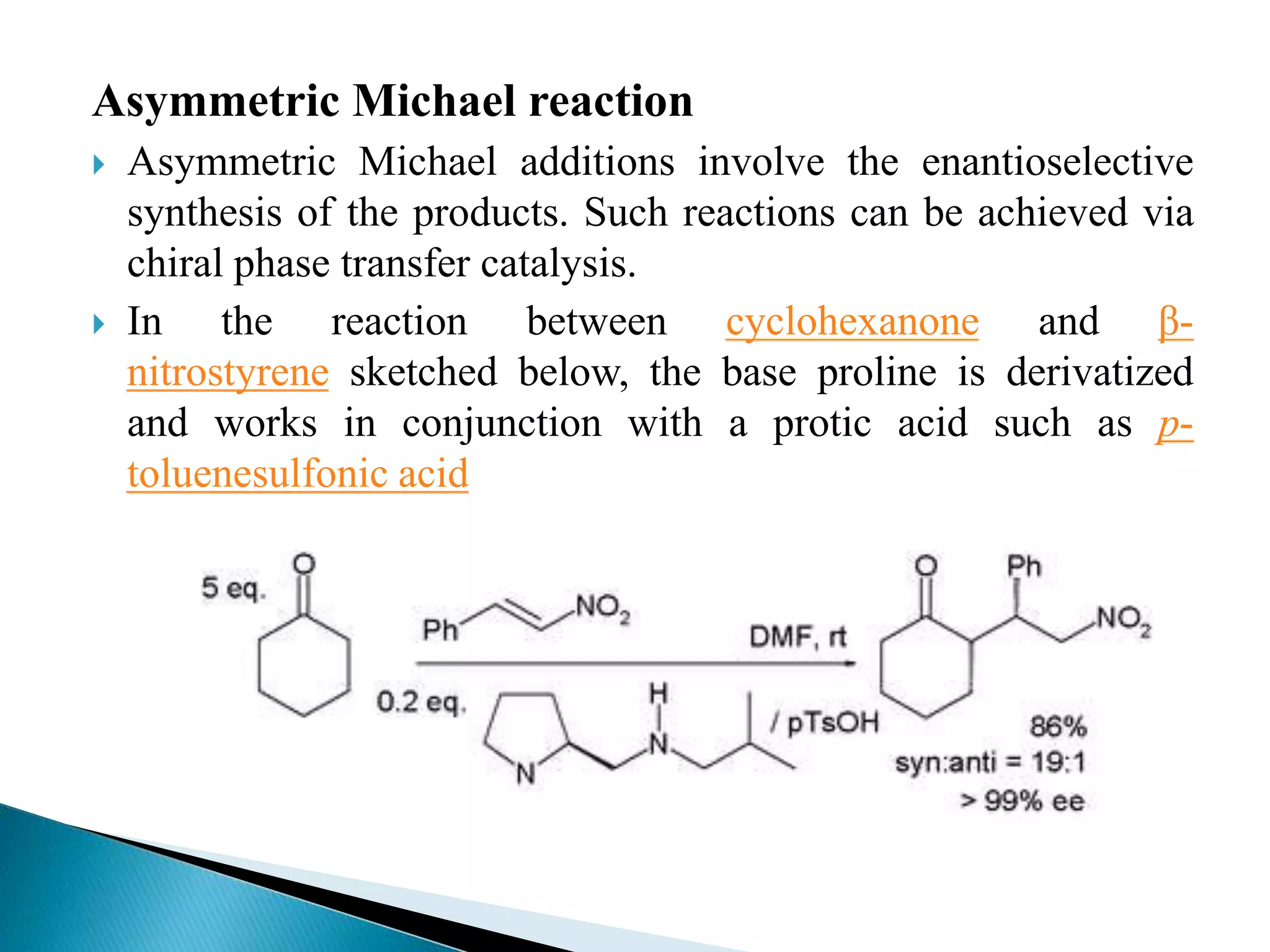
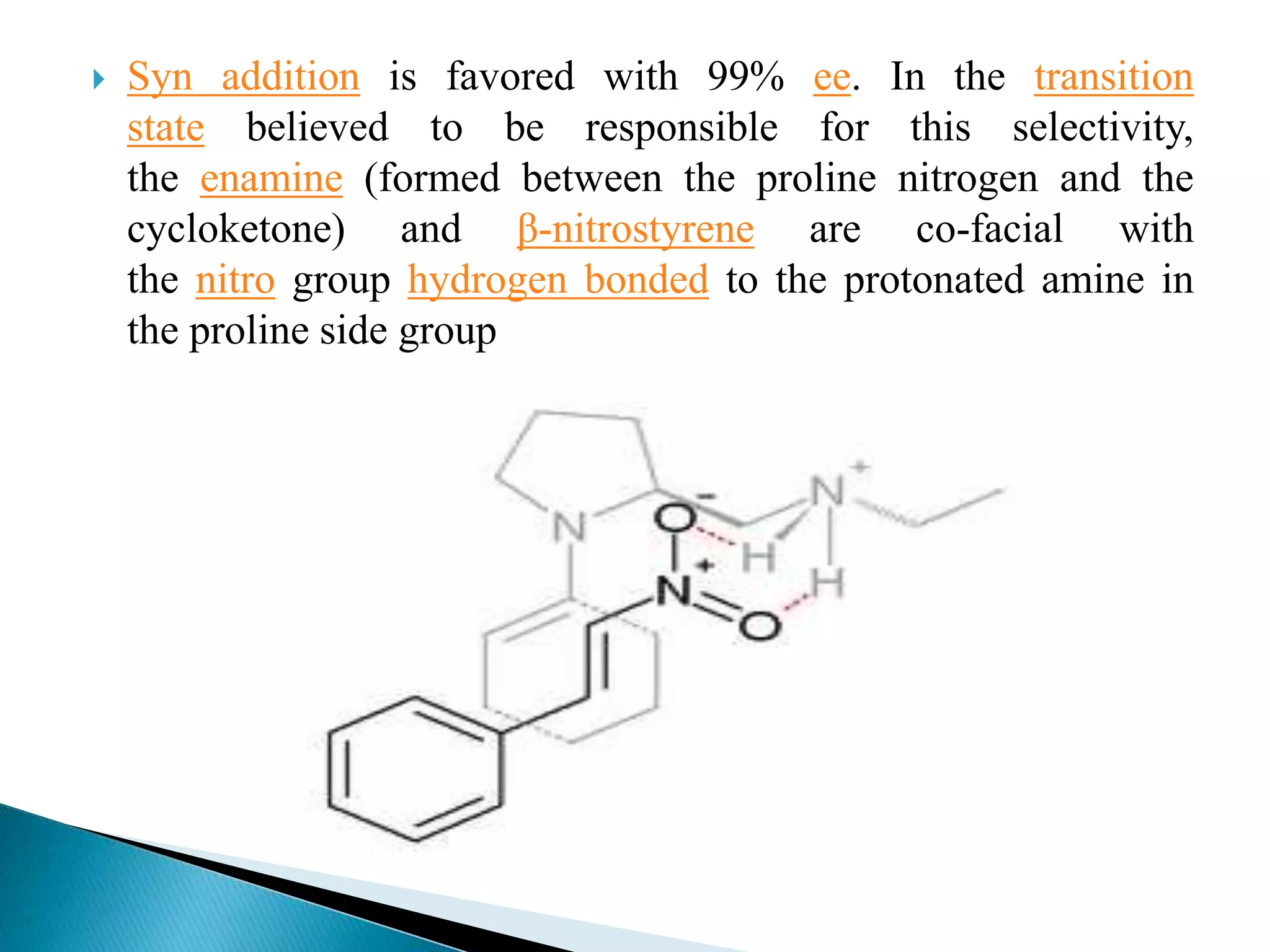
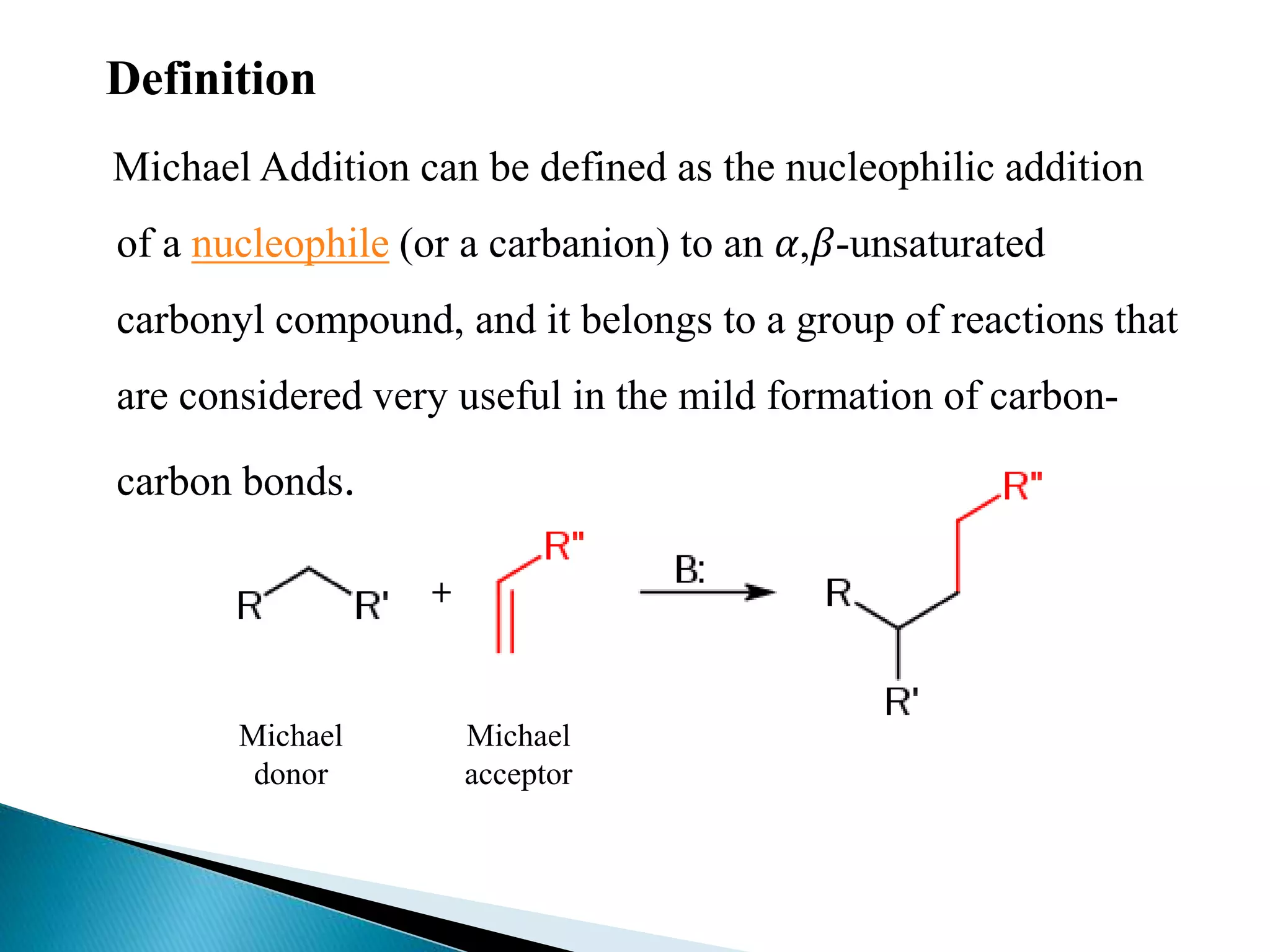
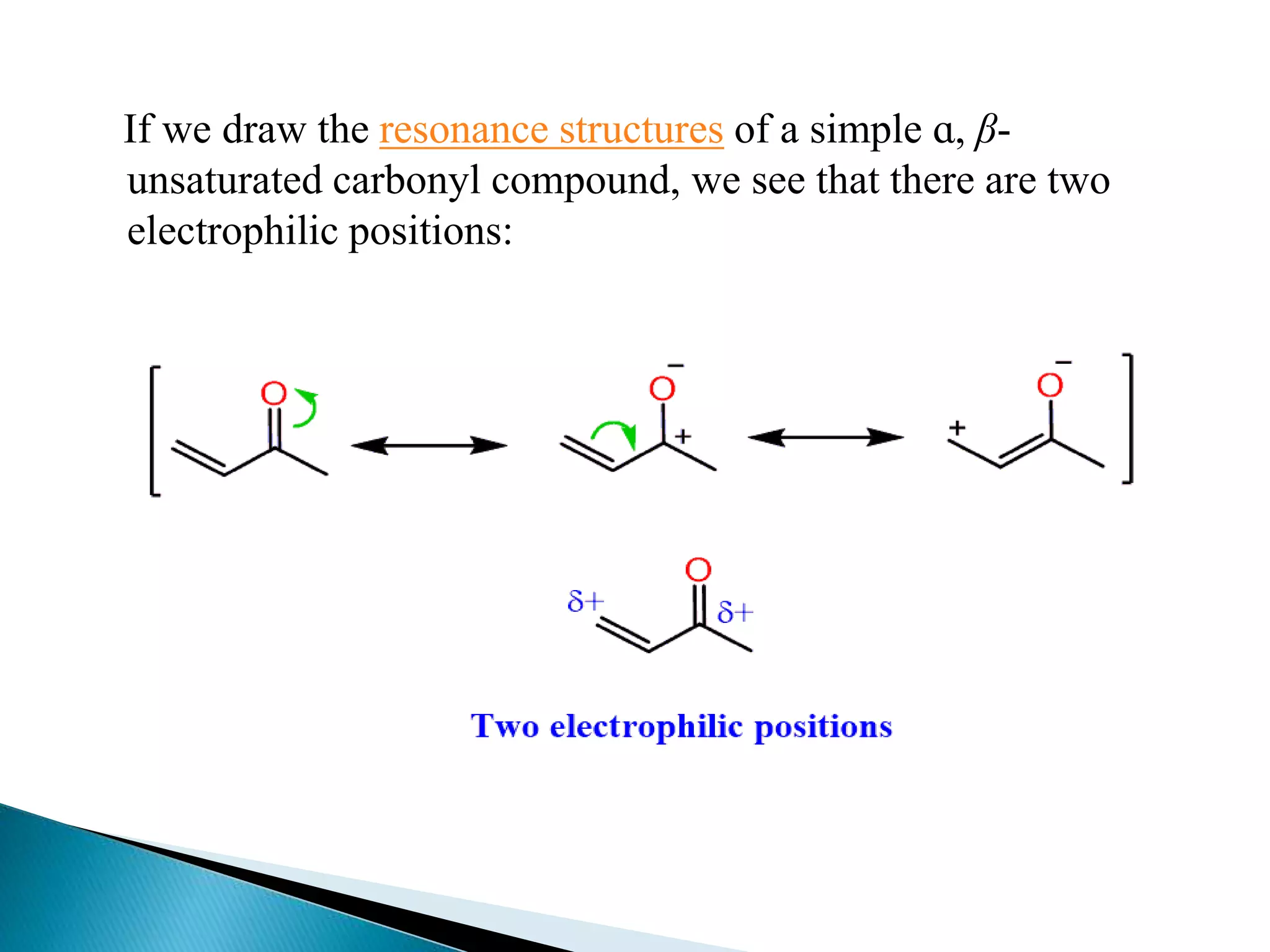
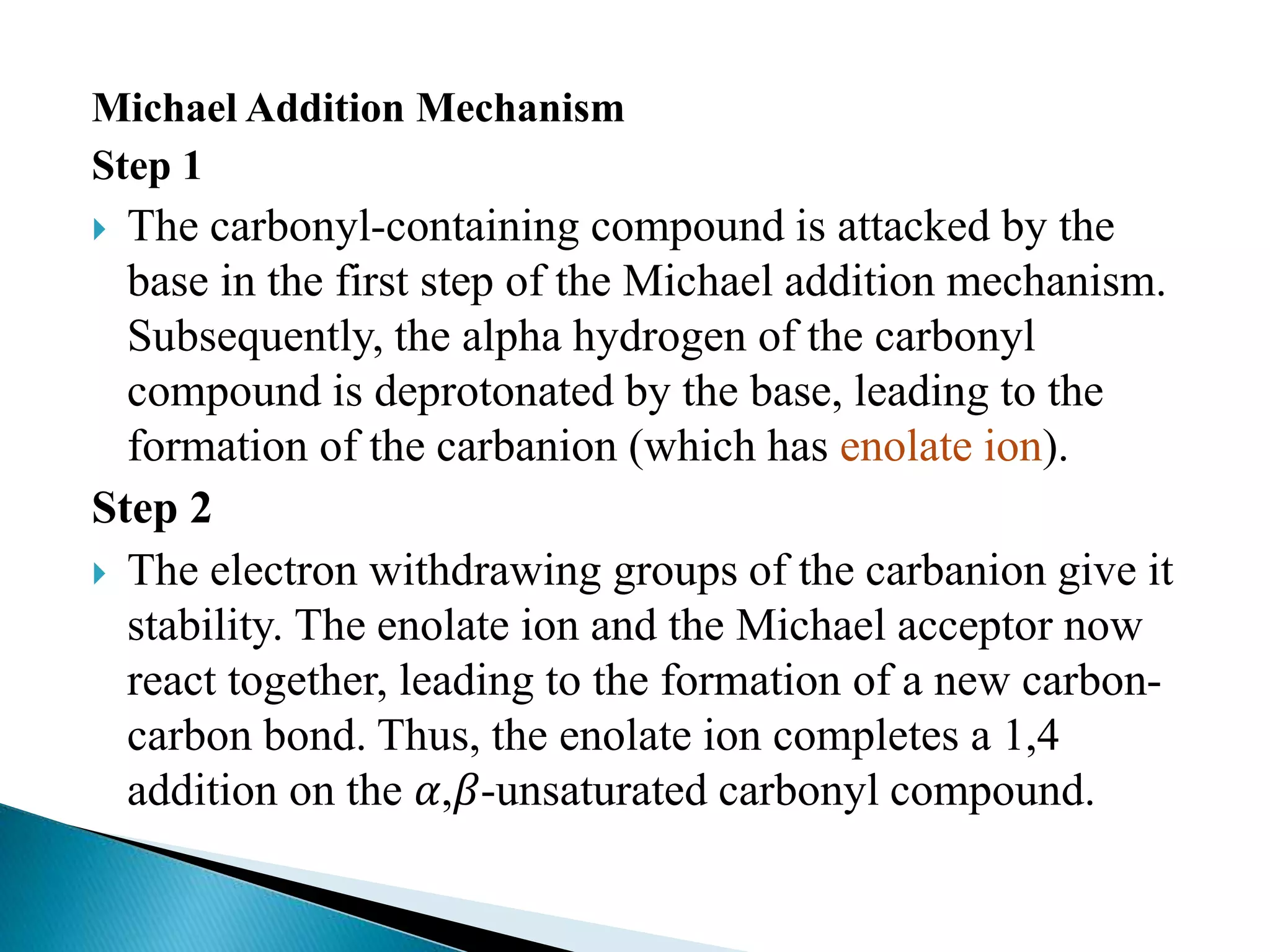
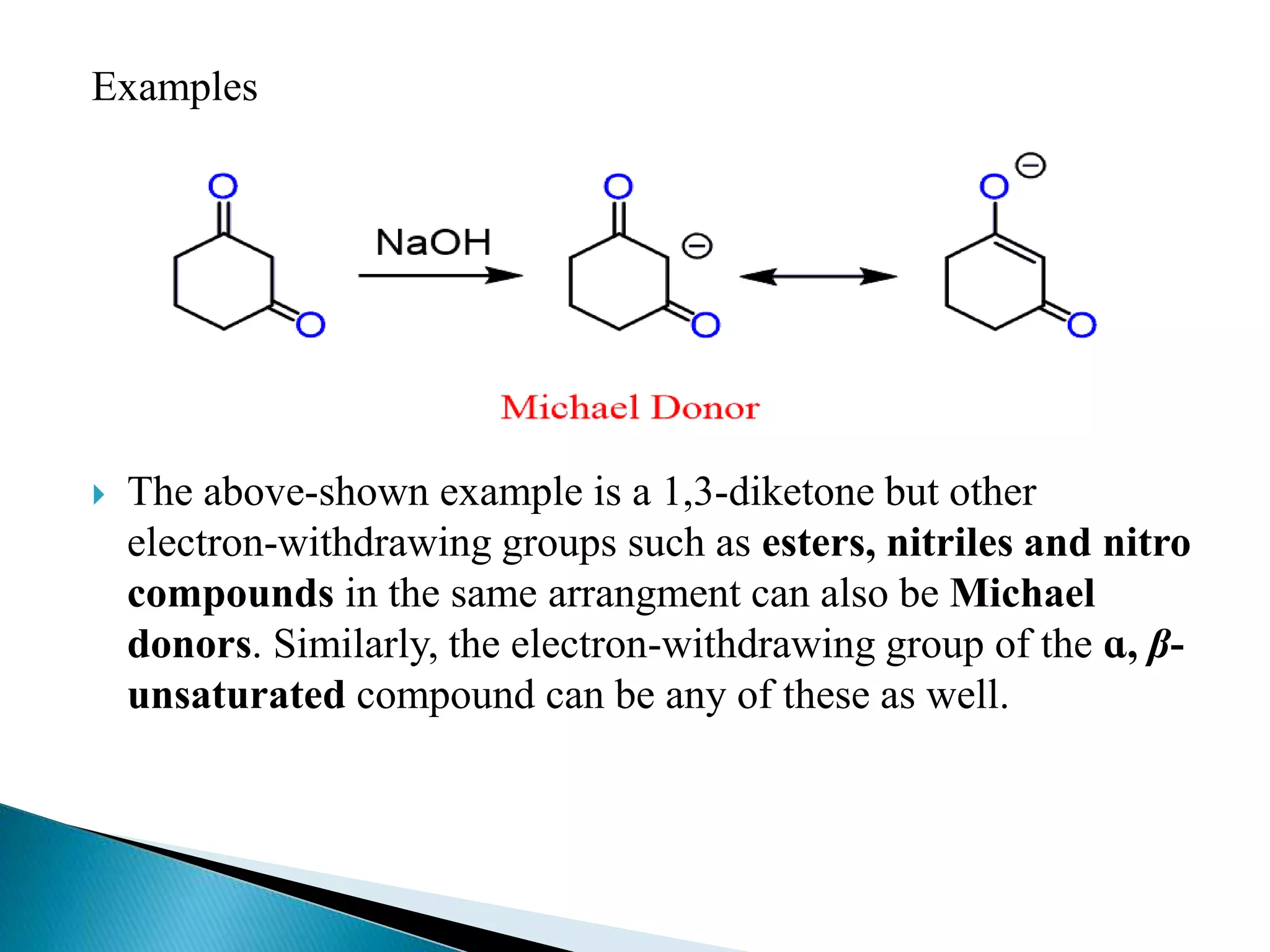
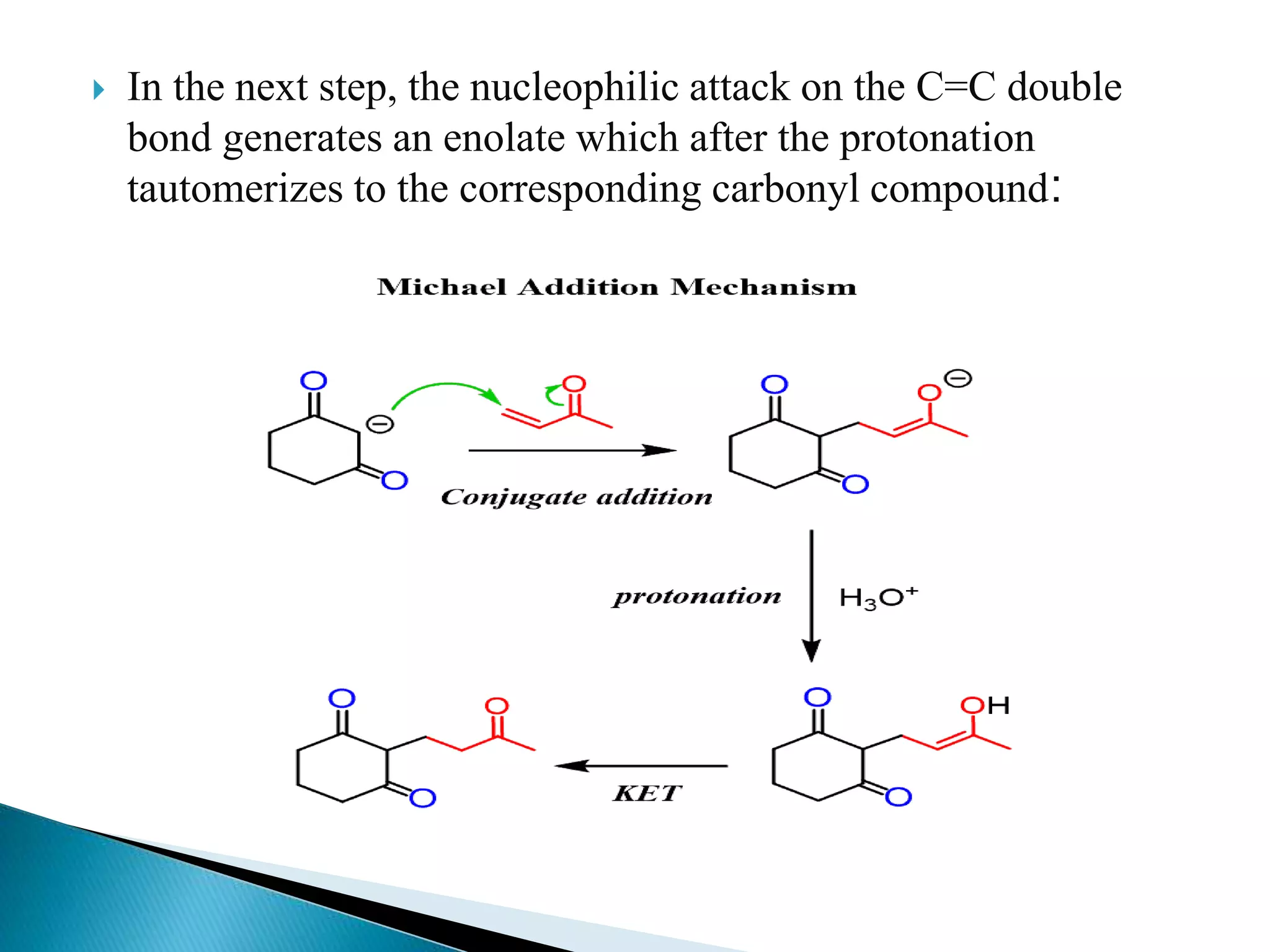
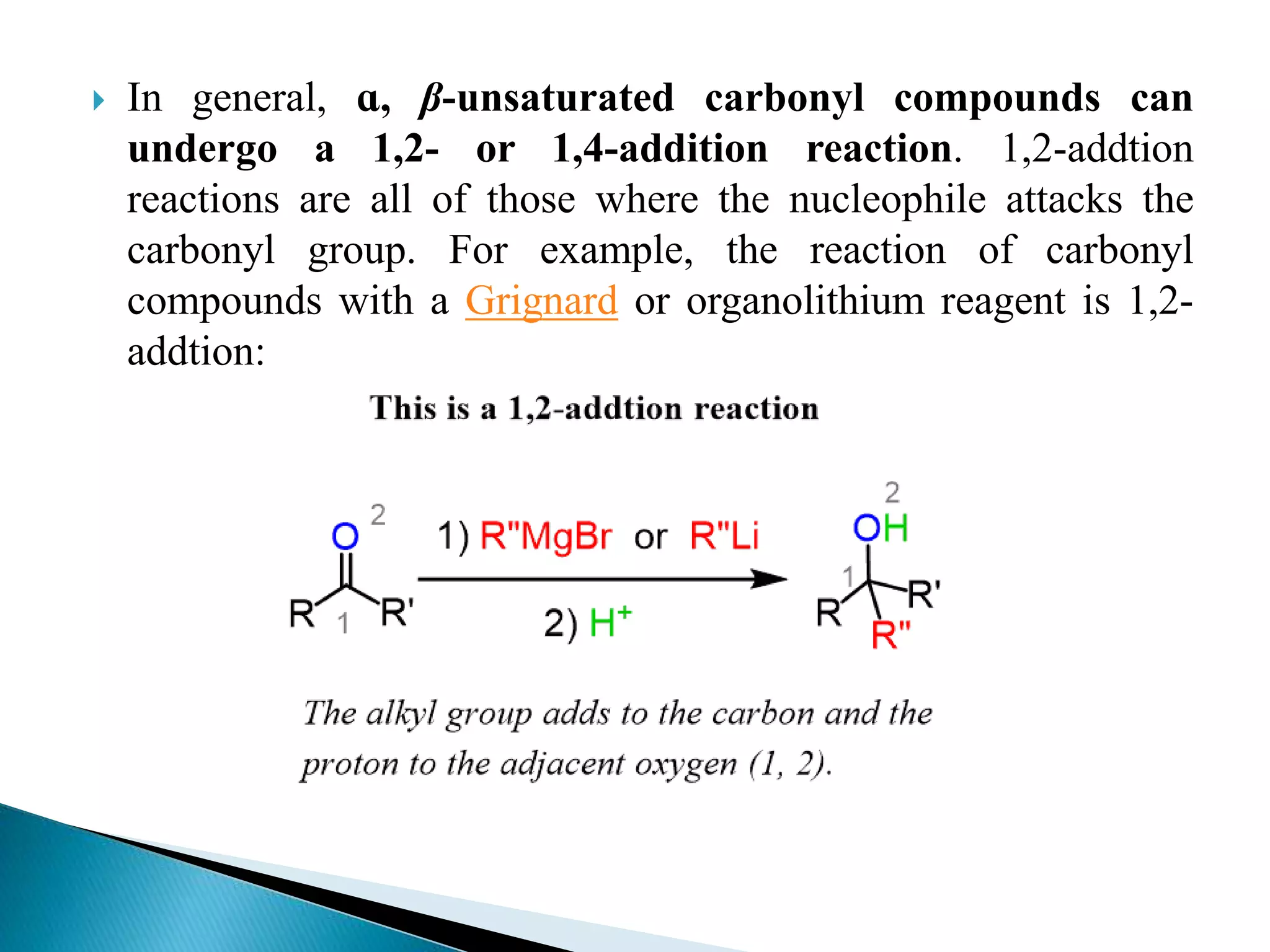
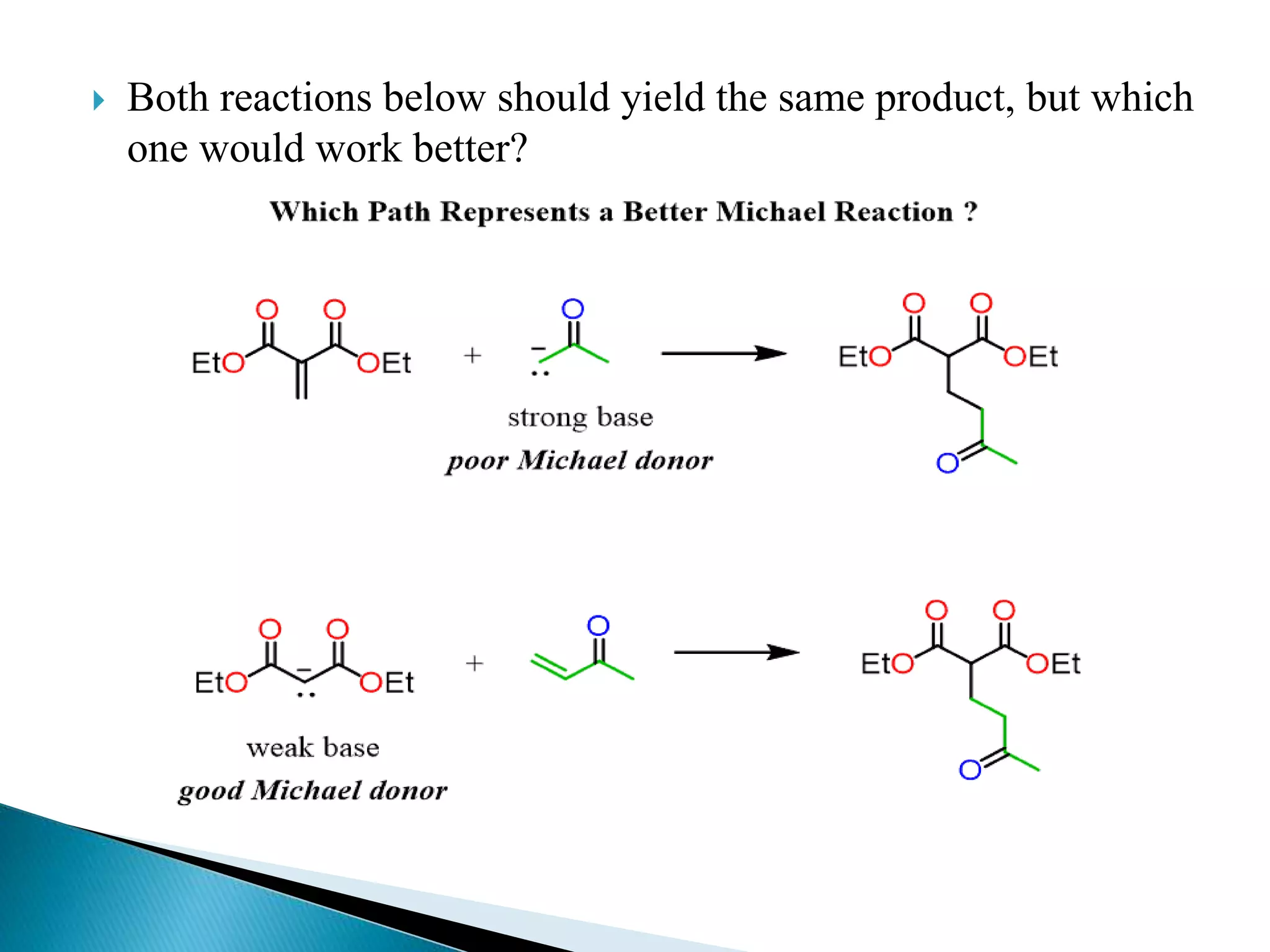
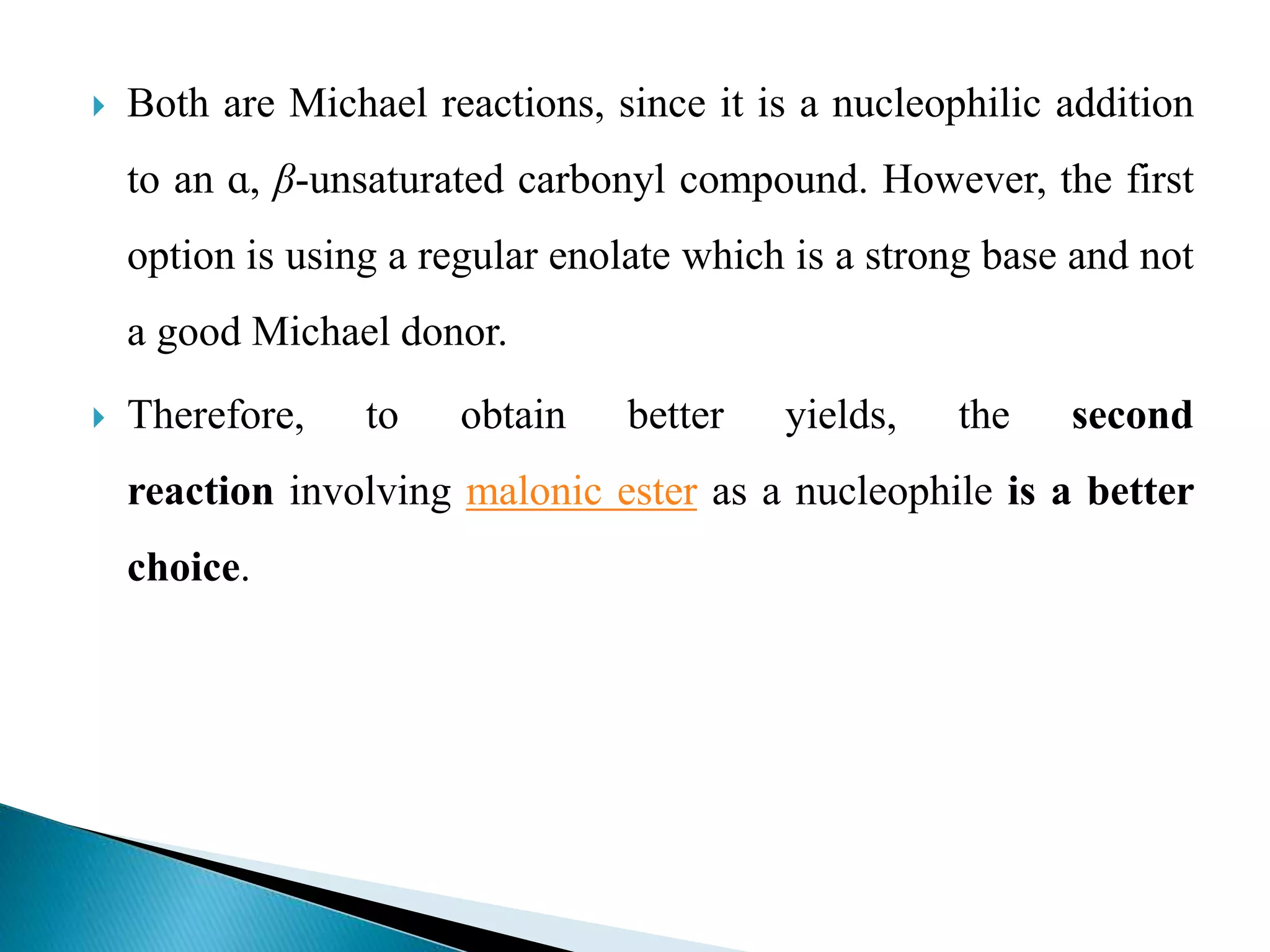
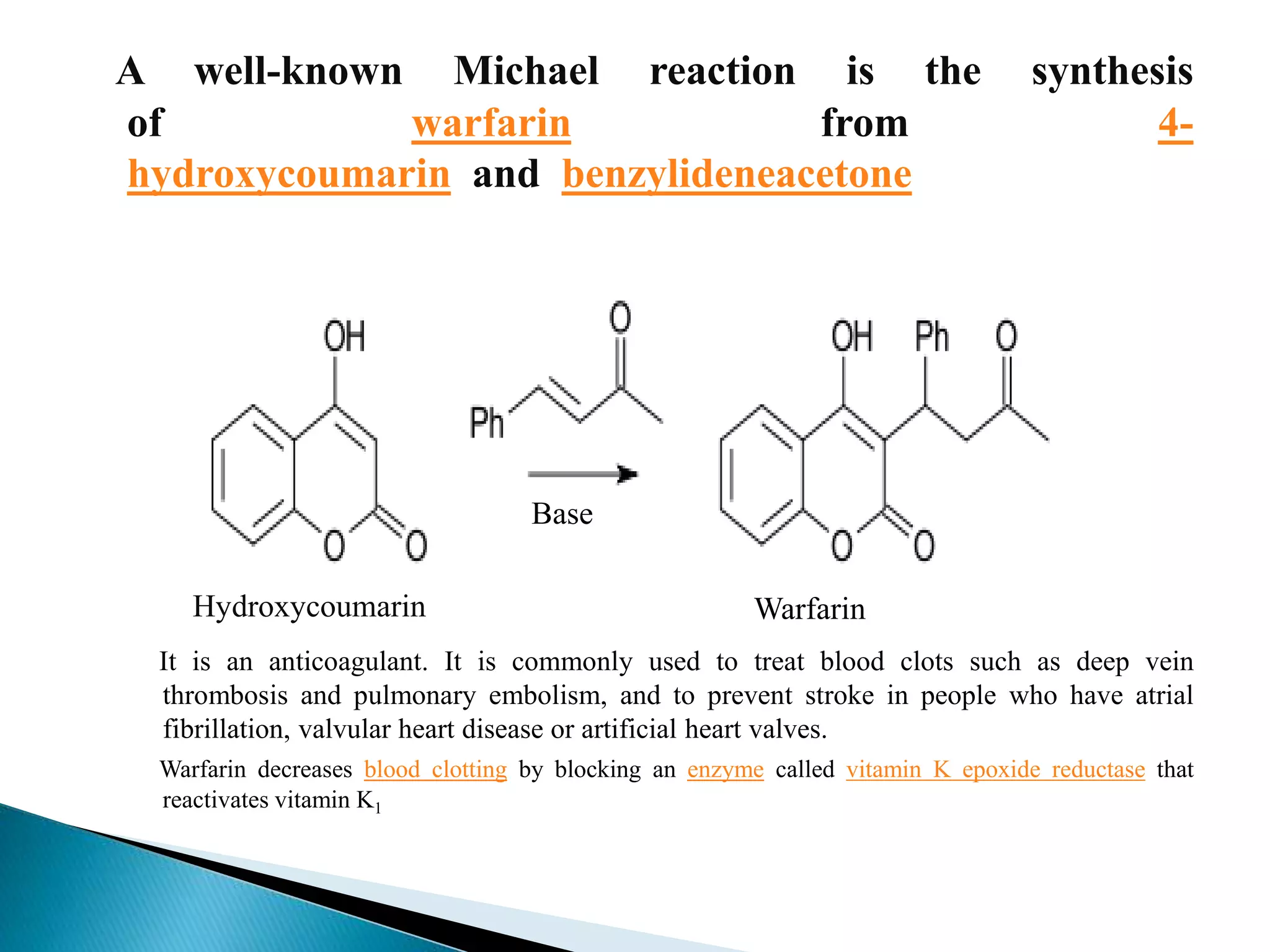
The document discusses the Michael addition reaction, which involves the nucleophilic addition of a carbanion to an α,β-unsaturated carbonyl compound. It provides the definition, mechanism, examples including the synthesis of warfarin, and applications such as asymmetric Michael reactions. The mechanism involves deprotonation of the carbonyl compound by a base to form an enolate ion, which adds to the Michael acceptor to form a new carbon-carbon bond via 1,4-addition.







![Example
Diethyl Malonate to Methyl Acrylates
diethyl [2-
(methoxycarbonyl)et
hyl]malonate
diethyl bis[2-
(methoxycarbonyl)ethyl]malo
nate](https://image.slidesharecdn.com/michaeladditionreaction1-210223033131/75/Michael-addition-reaction-16-2048.jpg)
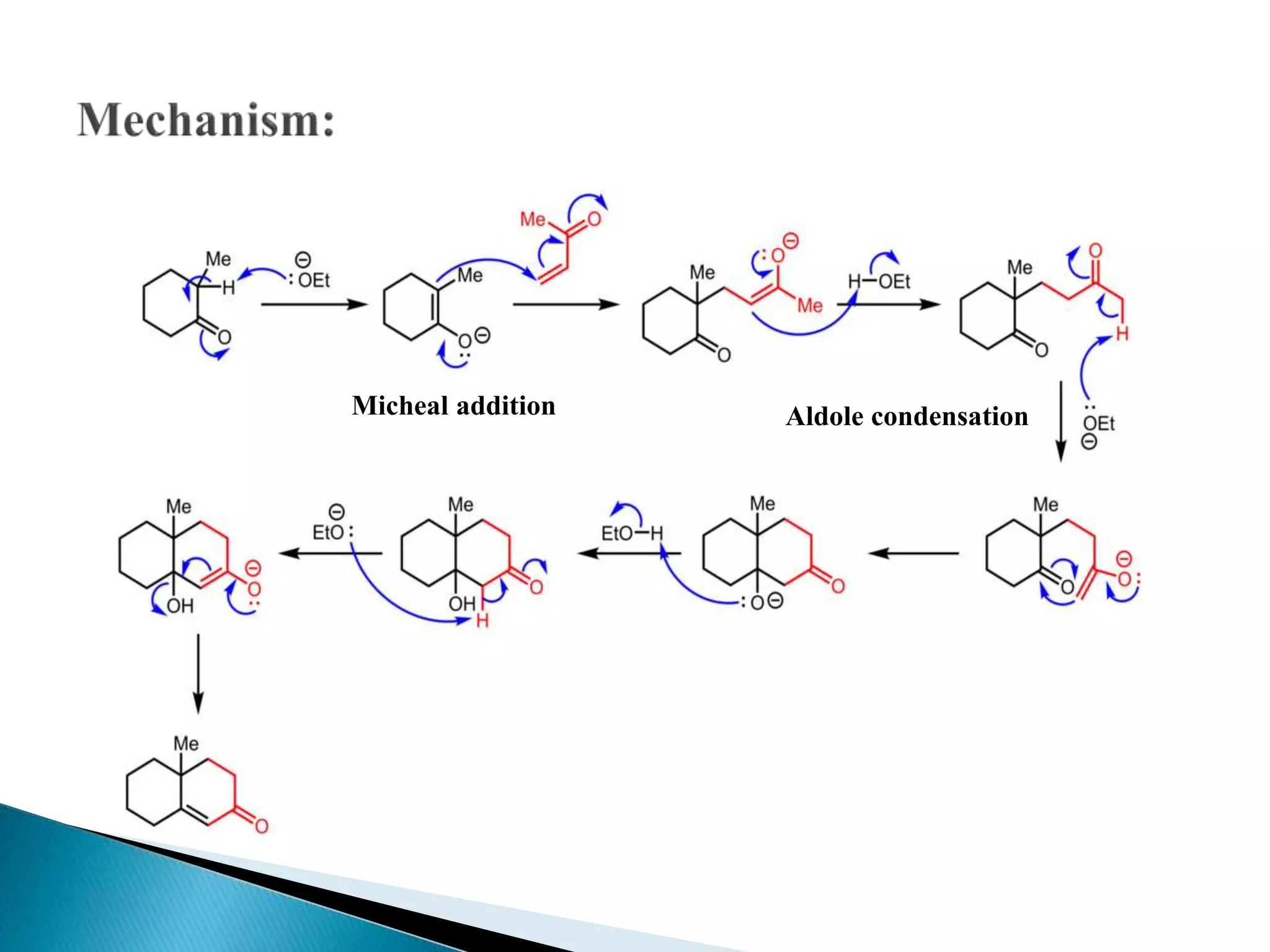
![ Robinson annulation
The Robinson annulation is a chemical reaction used in organic
chemistry for ring formation. It was discovered by Robert Robinson in
1935 as a method to create a six membered ring by forming three new
carbon–carbon bonds.[1] The method uses a ketone and a methyl vinyl
ketone to form an α,β-unsaturated ketone in a cyclohexane ring by
a Michael addition followed by an aldol condensation. This procedure is
one of the key methods to form fused ring systems](https://image.slidesharecdn.com/michaeladditionreaction1-210223033131/75/Michael-addition-reaction-19-2048.jpg)