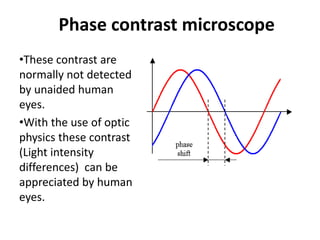
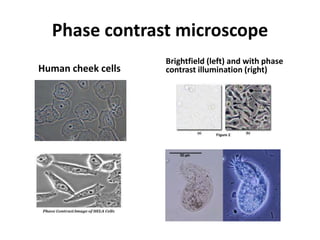
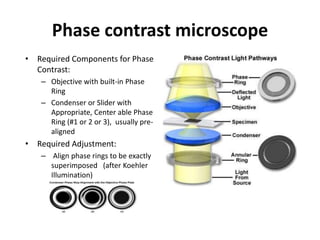
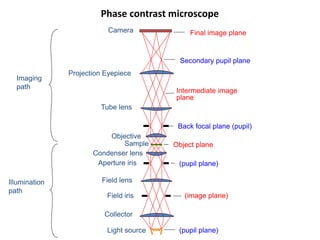
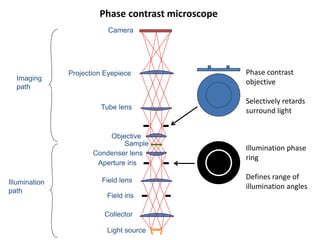
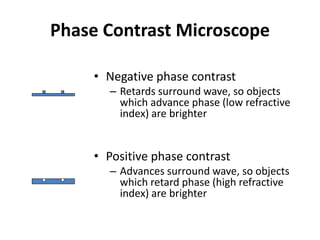
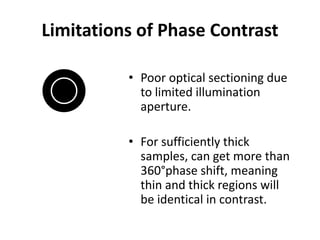
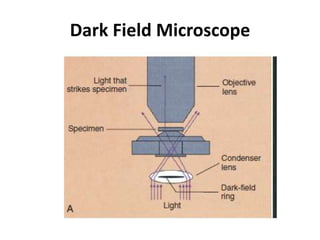
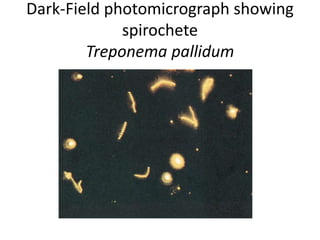
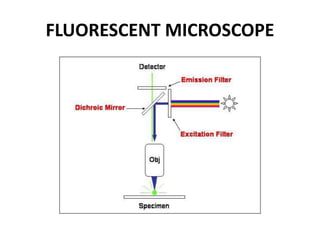
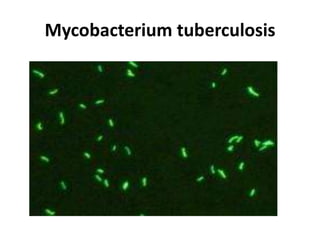
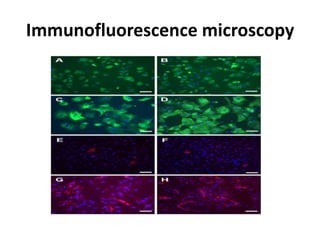
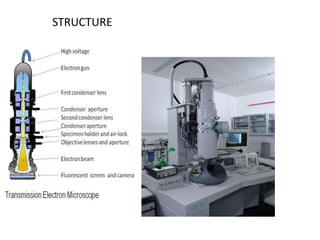
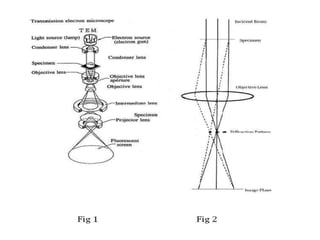
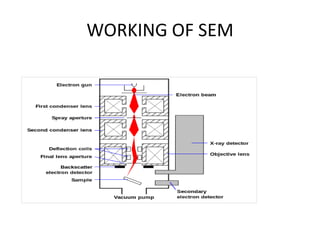
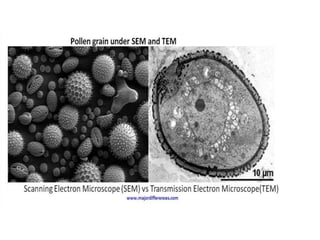
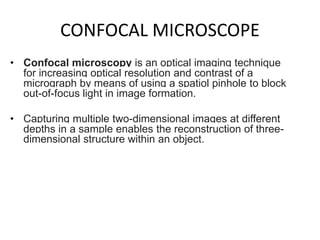
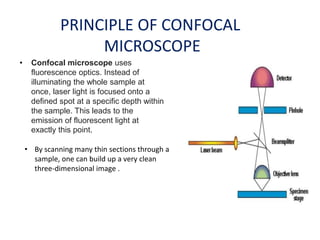
This document provides an overview of various types of microscopes and microscopy techniques. It discusses phase contrast microscopy, which uses differences in phase of light waves passing through a specimen to provide contrast for viewing unstained live samples. Darkfield microscopy is described as using oblique illumination to view small particles against a dark background. Fluorescent microscopy involves staining specimens with fluorescent dyes and using ultraviolet light to view the fluorescent emissions. Finally, electron microscopes are able to achieve higher resolutions than light microscopes by using electron beams rather than visible light. Transmission electron microscopes transmit electrons through thin samples to form images.