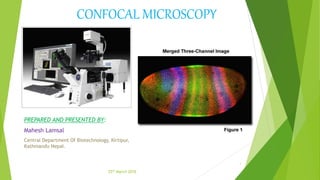
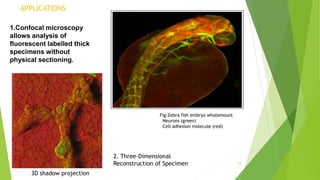
The document provides an overview of confocal microscopy. It discusses the history, starting with Minsky's invention of the confocal microscope in 1957. The instrumental design uses a pinhole to reject out-of-focus light and produce optical sections through a specimen. The principle involves illuminating a point on the specimen with a laser and detecting the resulting fluorescence through a pinhole, rejecting out-of-focus light. Applications include analyzing thick fluorescent specimens, 3D reconstruction, and improved resolution over conventional microscopy. Advantages are uniform illumination and better optical sections while limitations include resolution and photobleaching.