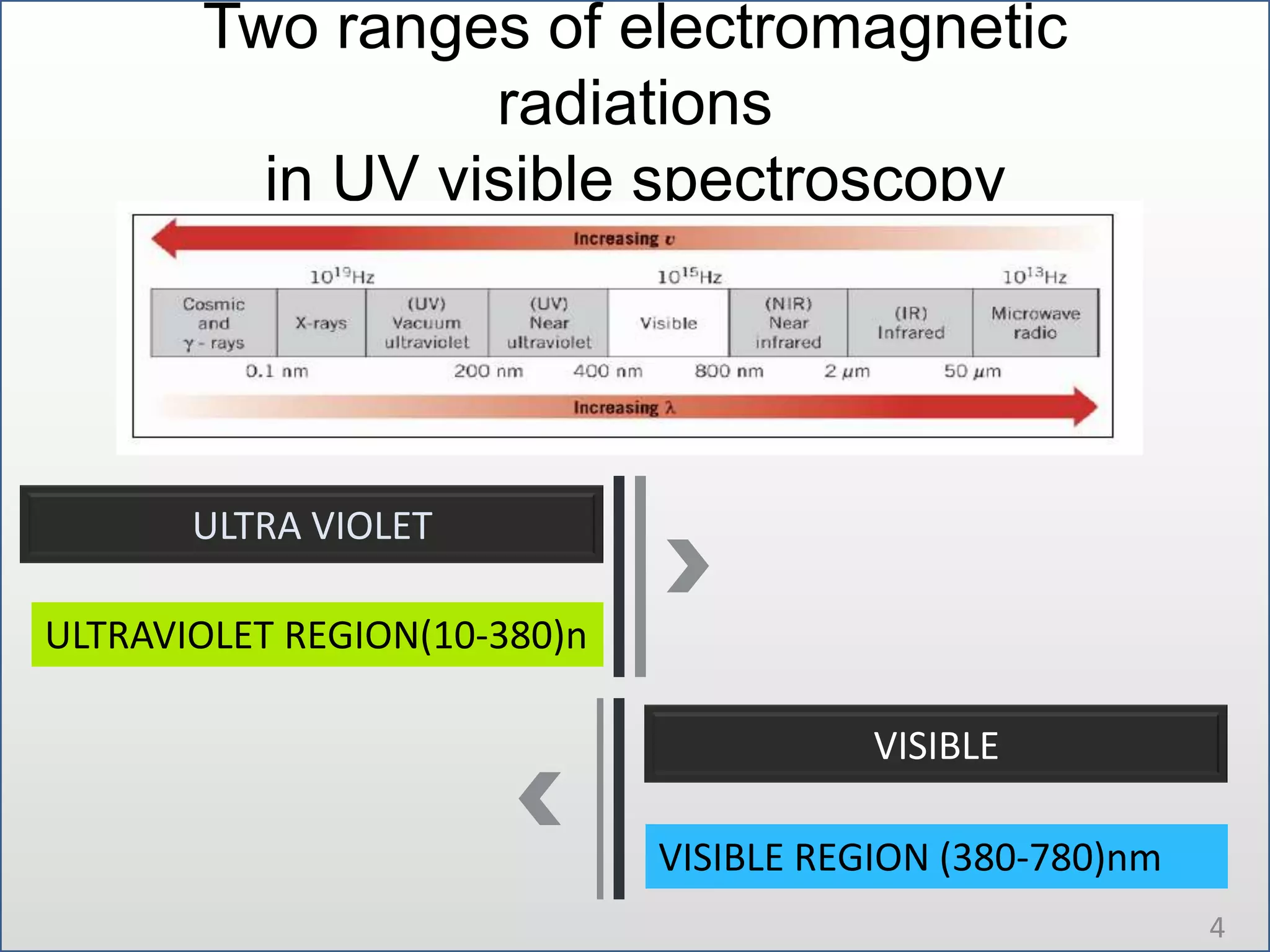
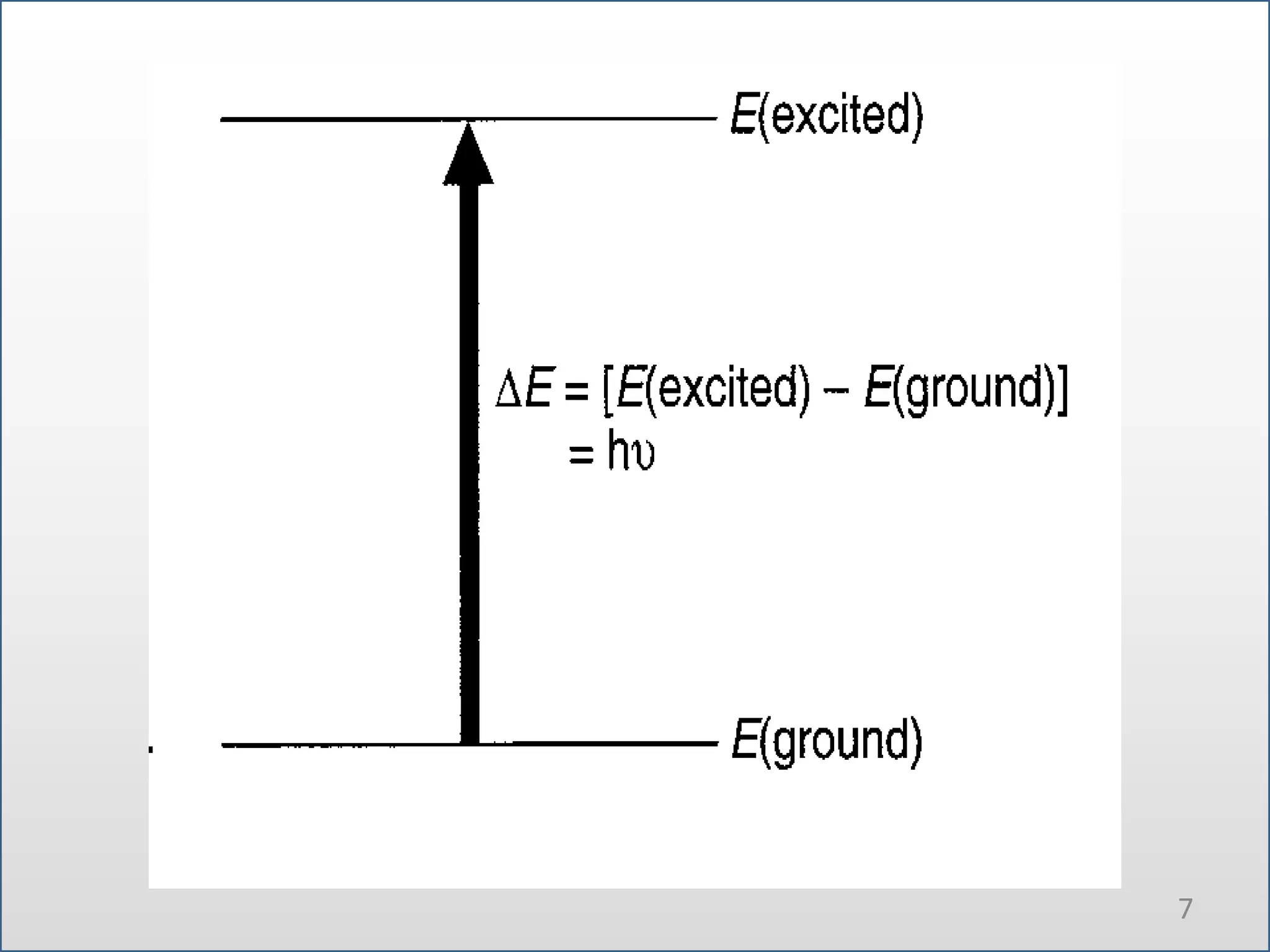
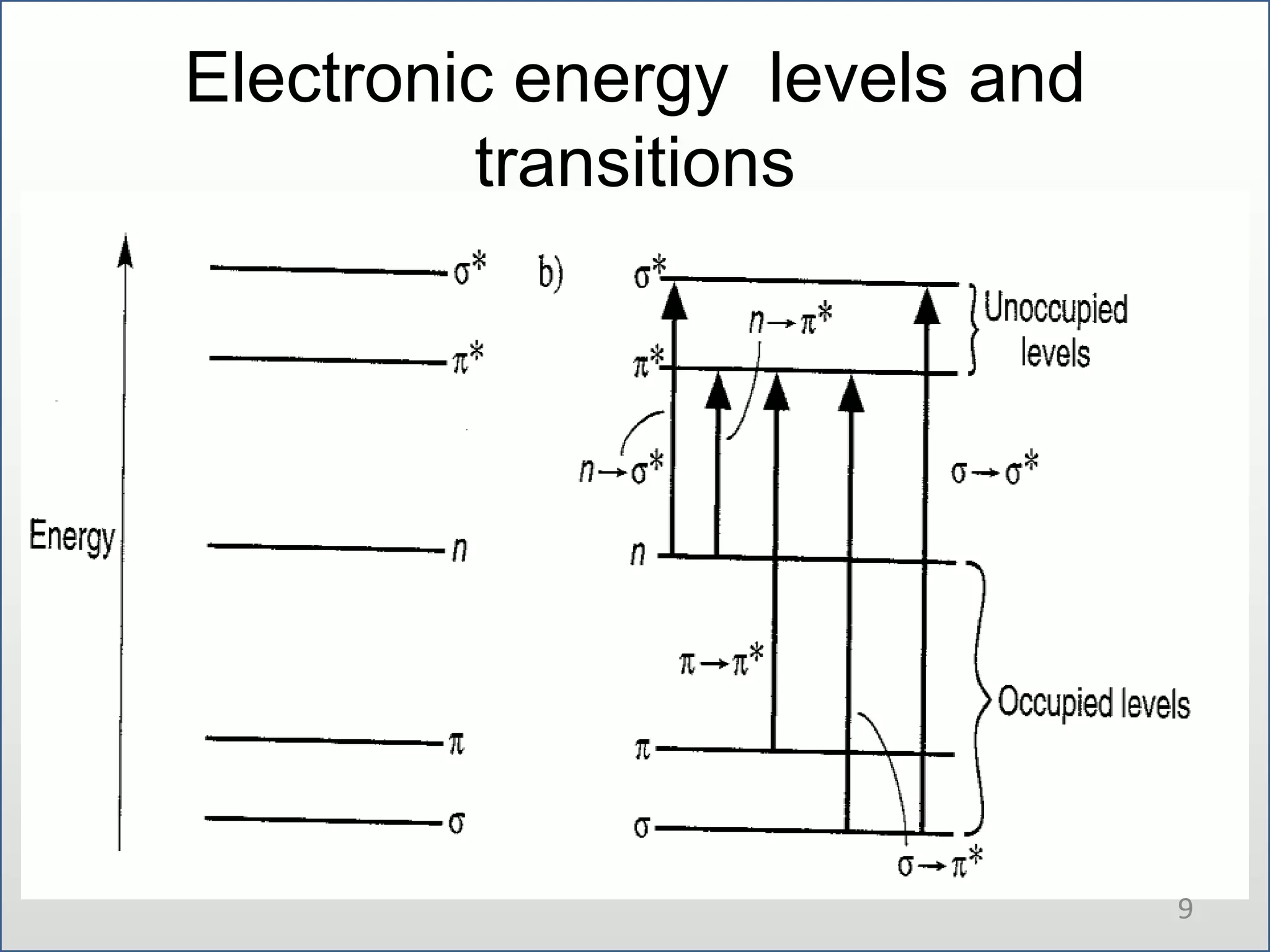
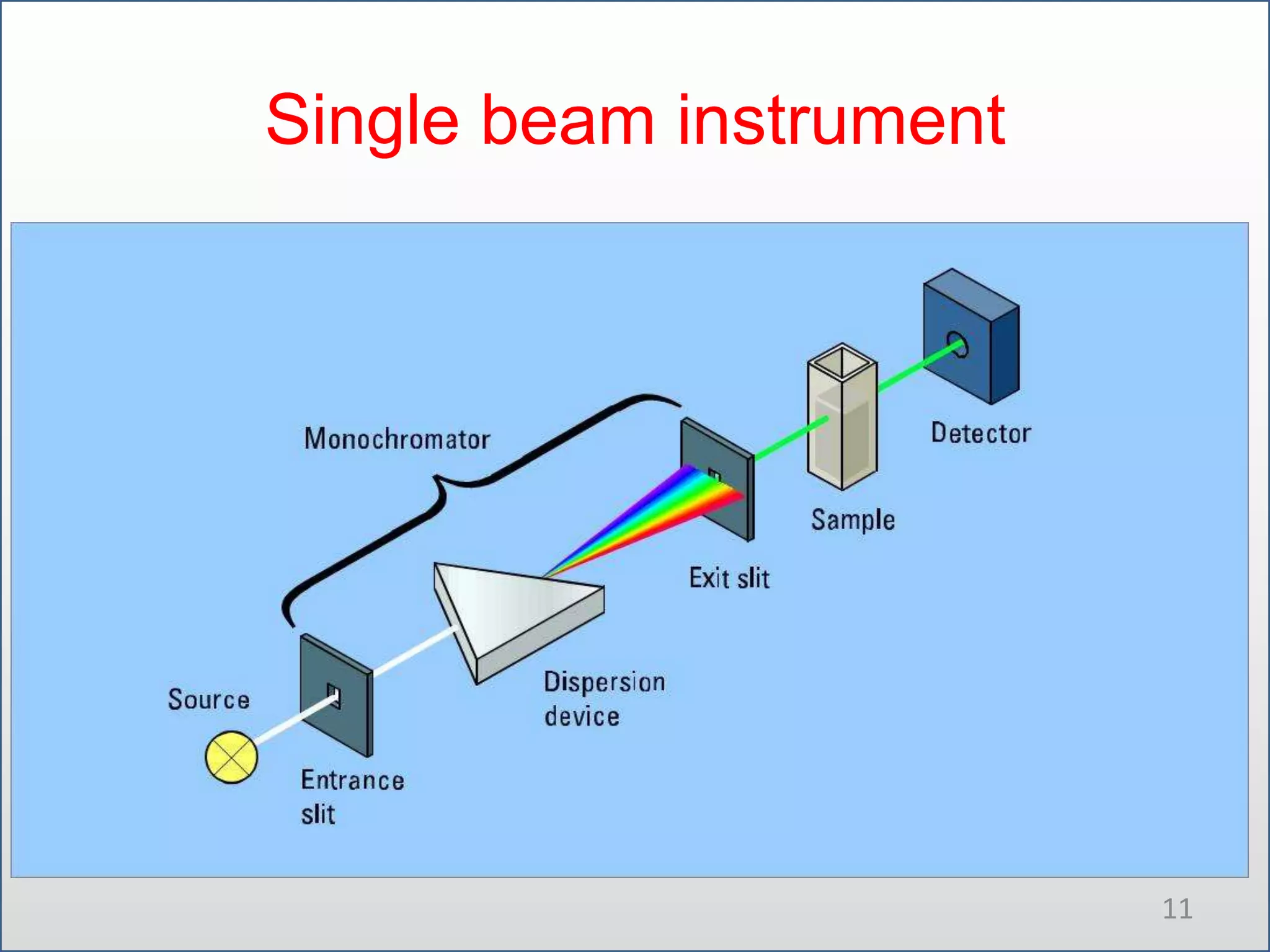
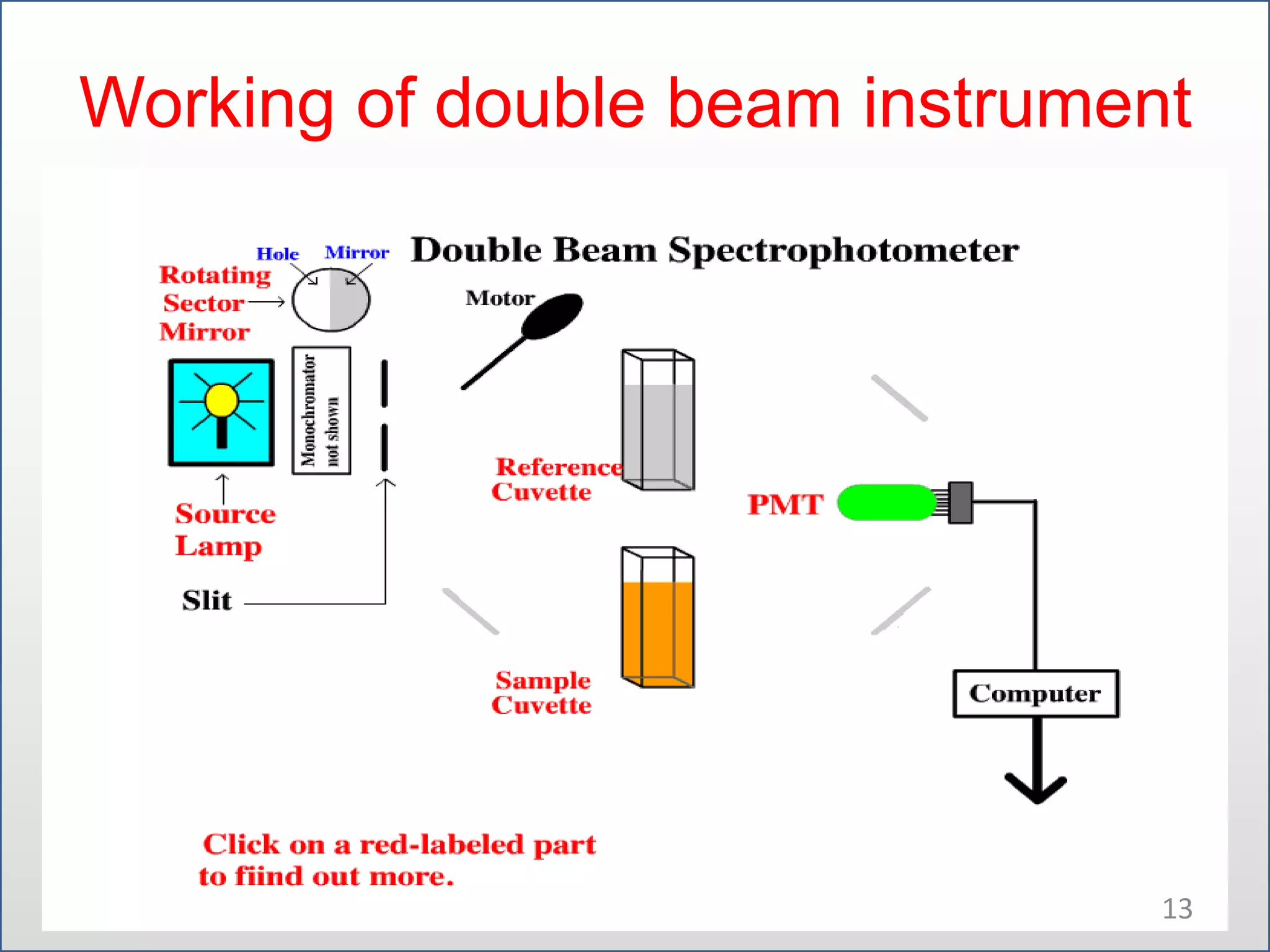
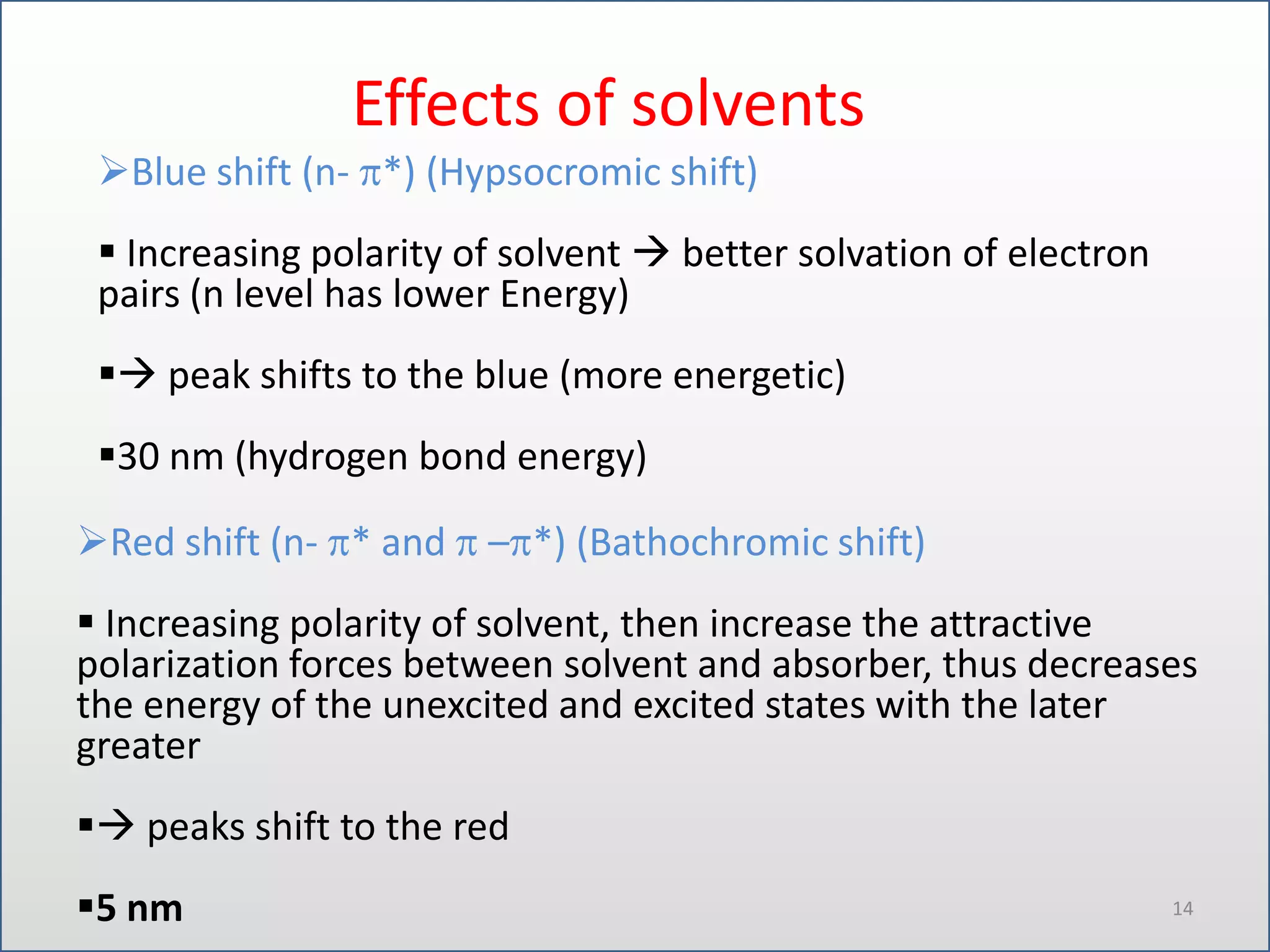
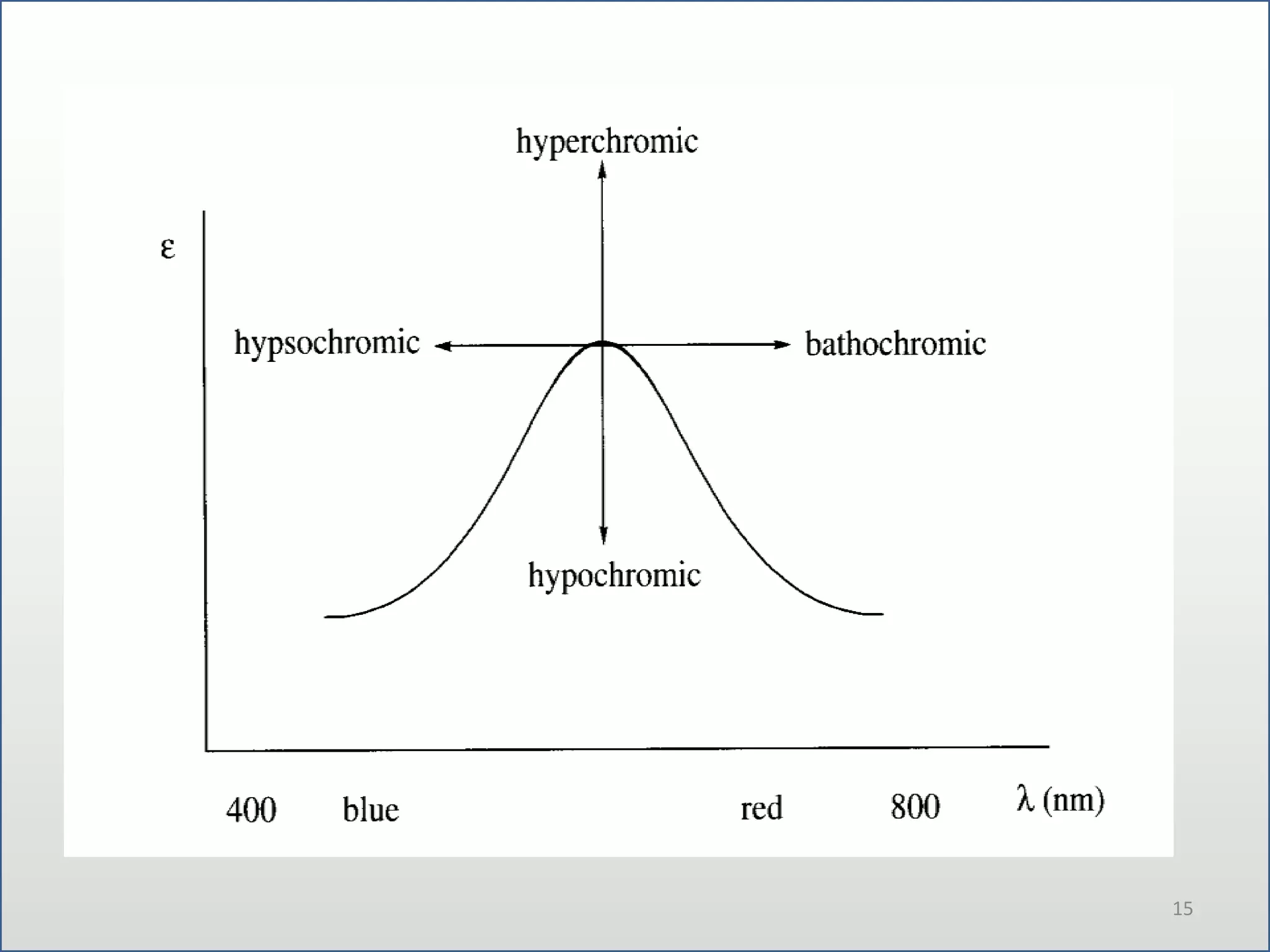
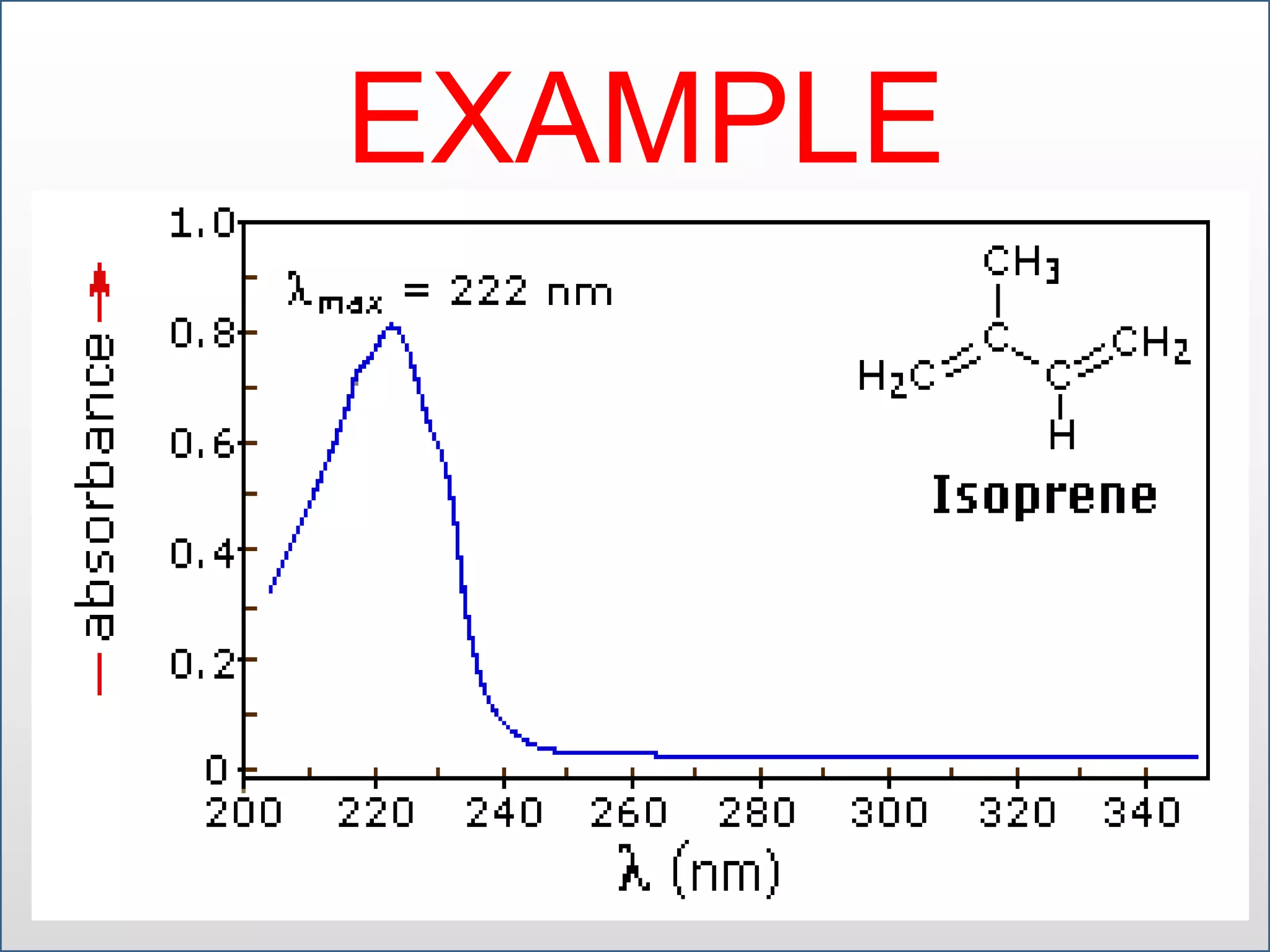
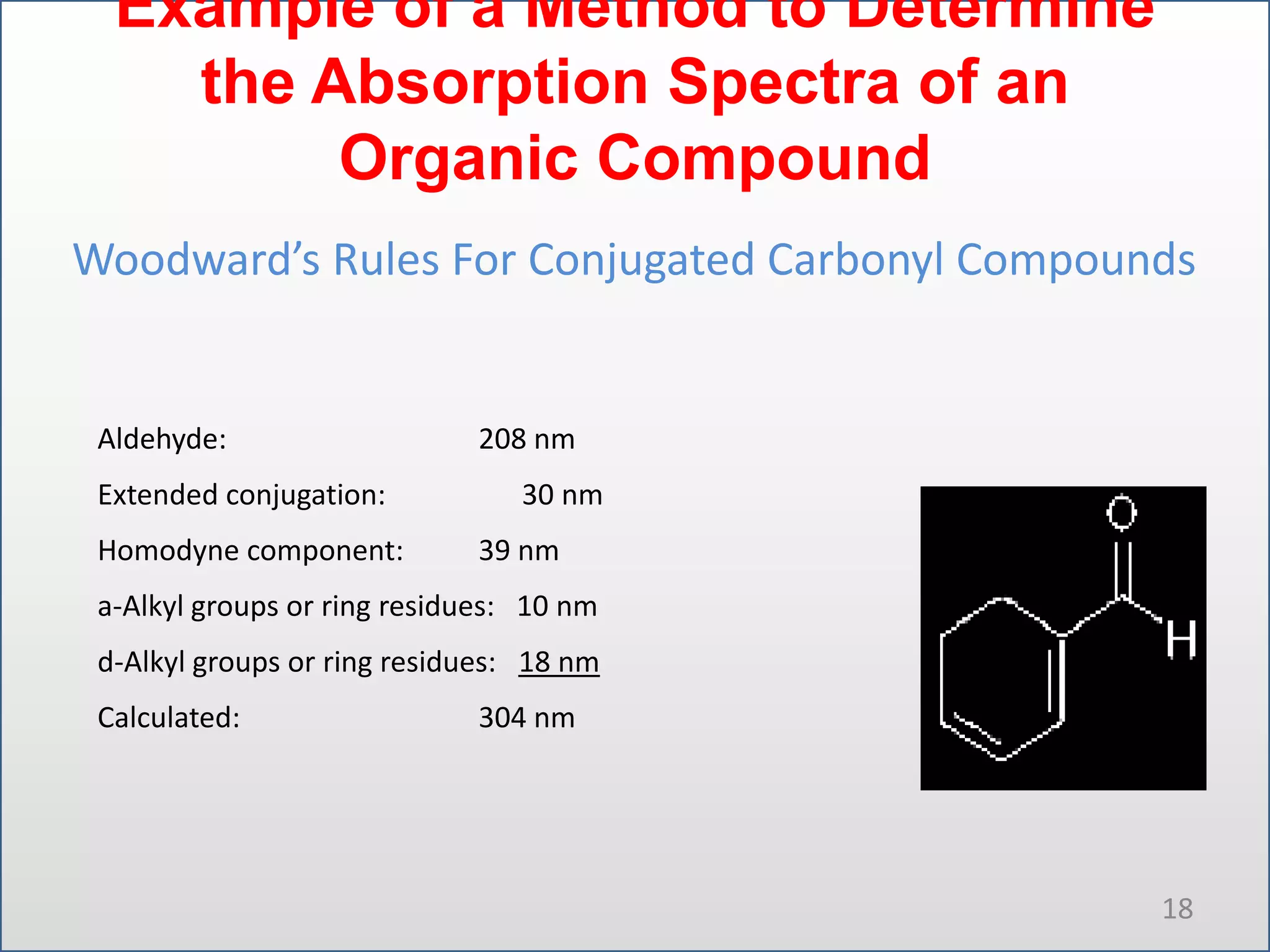
UV-visible spectroscopy involves measuring the amount of light absorbed by a molecule at different wavelengths in the UV and visible regions. There are two main regions - the UV region from 10-380nm and the visible region from 380-780nm. The technique works by exciting electrons from occupied to unoccupied orbitals when molecules absorb radiation. There are four main types of electronic transitions that can occur. Instruments like single beam and double beam spectrophotometers are used to collect absorption spectra. Absorption maxima provide information about functional groups and can be used to determine structures and for analytical and stereochemical applications.