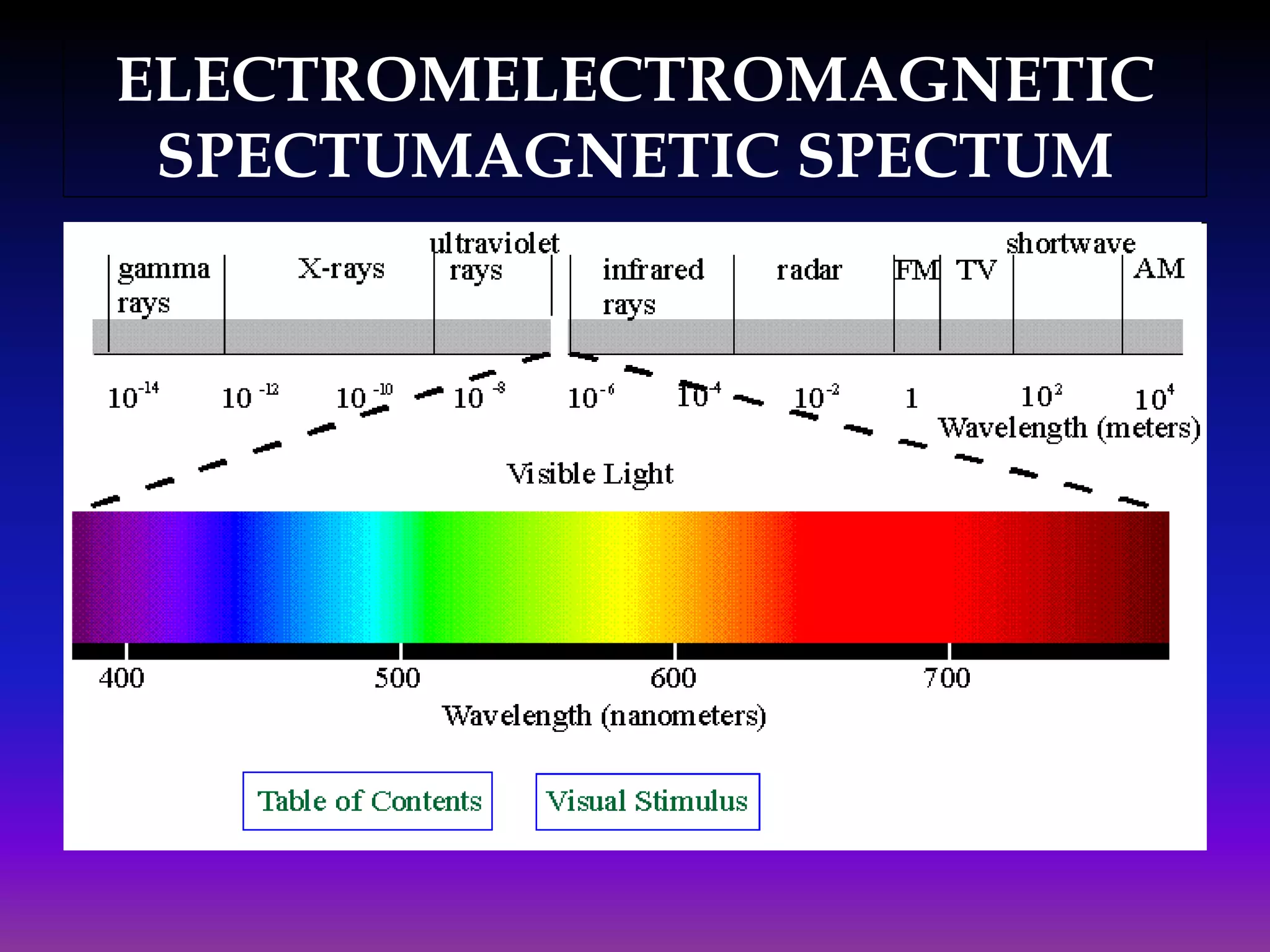
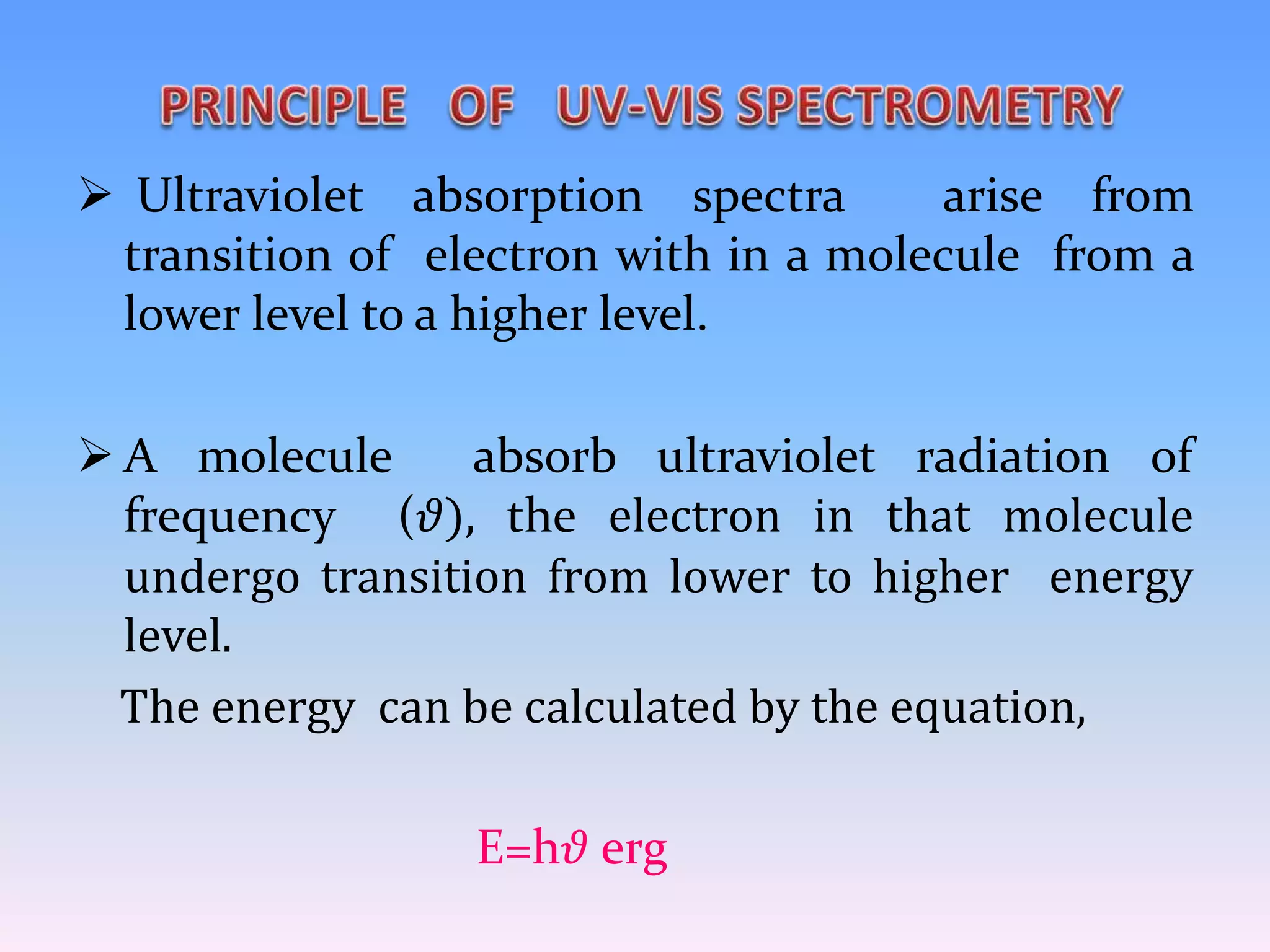
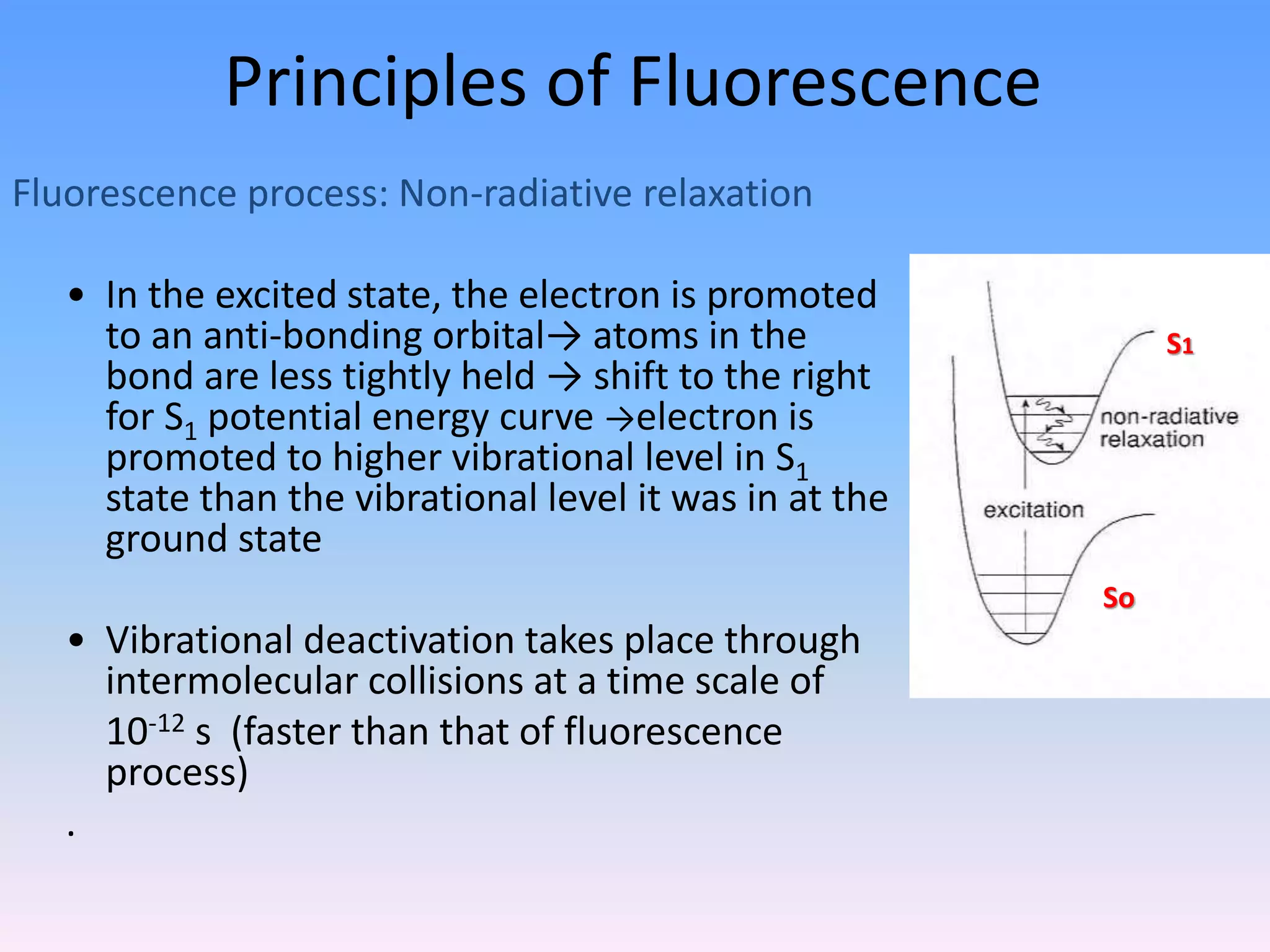
Ultraviolet and visible (UV-Vis) absorption spectroscopy measures the attenuation of light passing through or reflected from a sample. When light energy matches an electronic transition in a molecule, some light is absorbed, promoting electrons to higher orbitals. The resulting absorbance spectrum shows absorbance versus wavelength. Fluorescence spectroscopy involves excitation of molecules to higher electronic singlet states followed by emission of light as they relax to ground states. Quantum yield is the ratio of emitted to absorbed photons. Both techniques are useful in characterizing biological systems like proteins, DNA, and fluorophores.