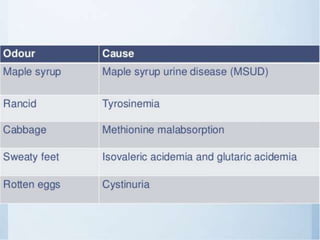
The document provides information about urine analysis, including its importance, the examination process, and various tests. It discusses:
- Urine analysis involves macroscopic, chemical, and microscopic examination to diagnose conditions.
- Tests include assessment of physical characteristics like volume, color, and specific gravity as well as chemical tests for proteins, sugars, ketones, blood, and bile.
- Proper collection and preservation methods are outlined to transport urine samples to the laboratory for analysis within 2 hours to avoid deterioration.
- Common chemical tests described are Benedict's test for sugars, Rothera's test for ketones, and heat and acetic acid test for proteins.