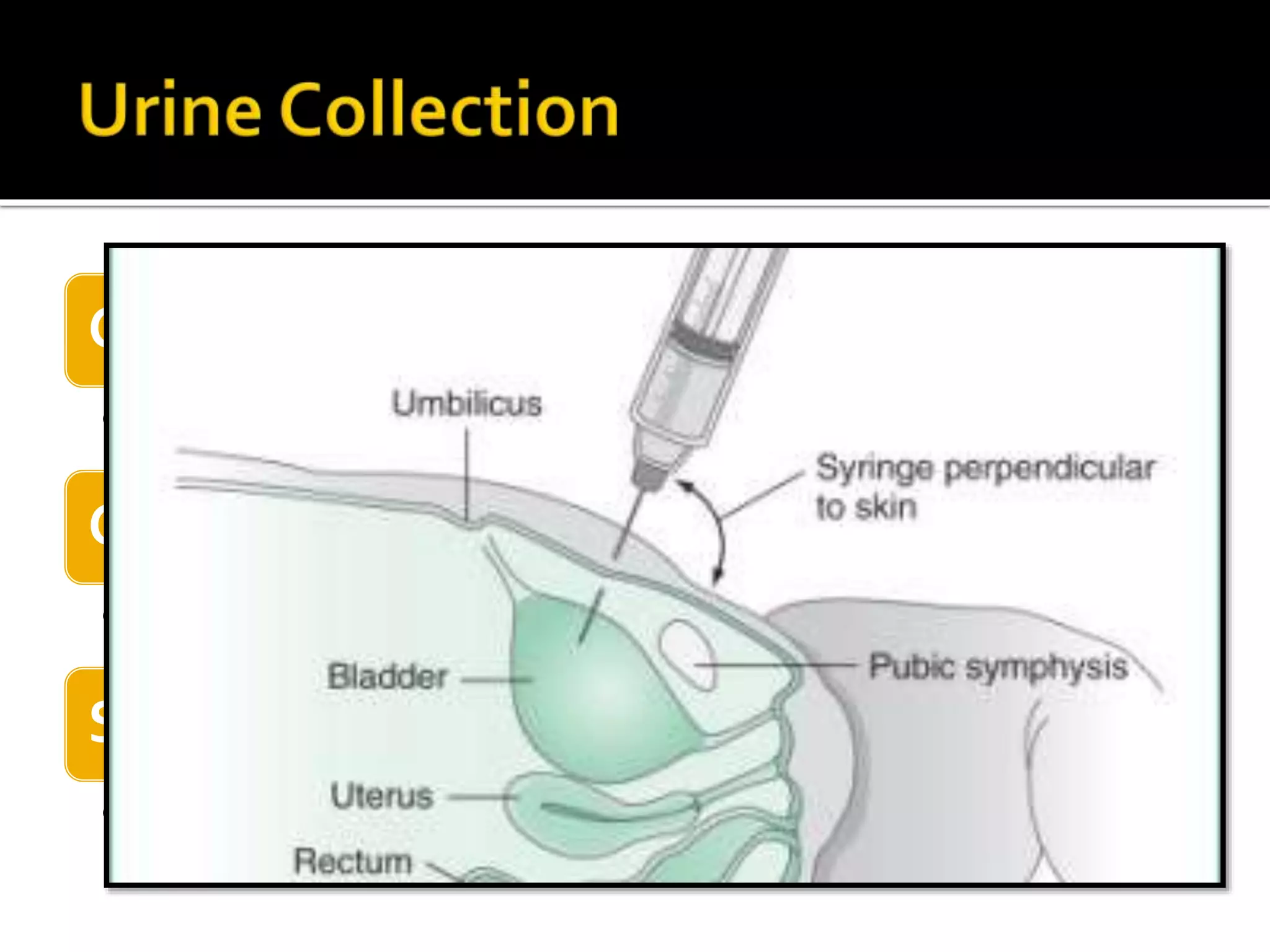
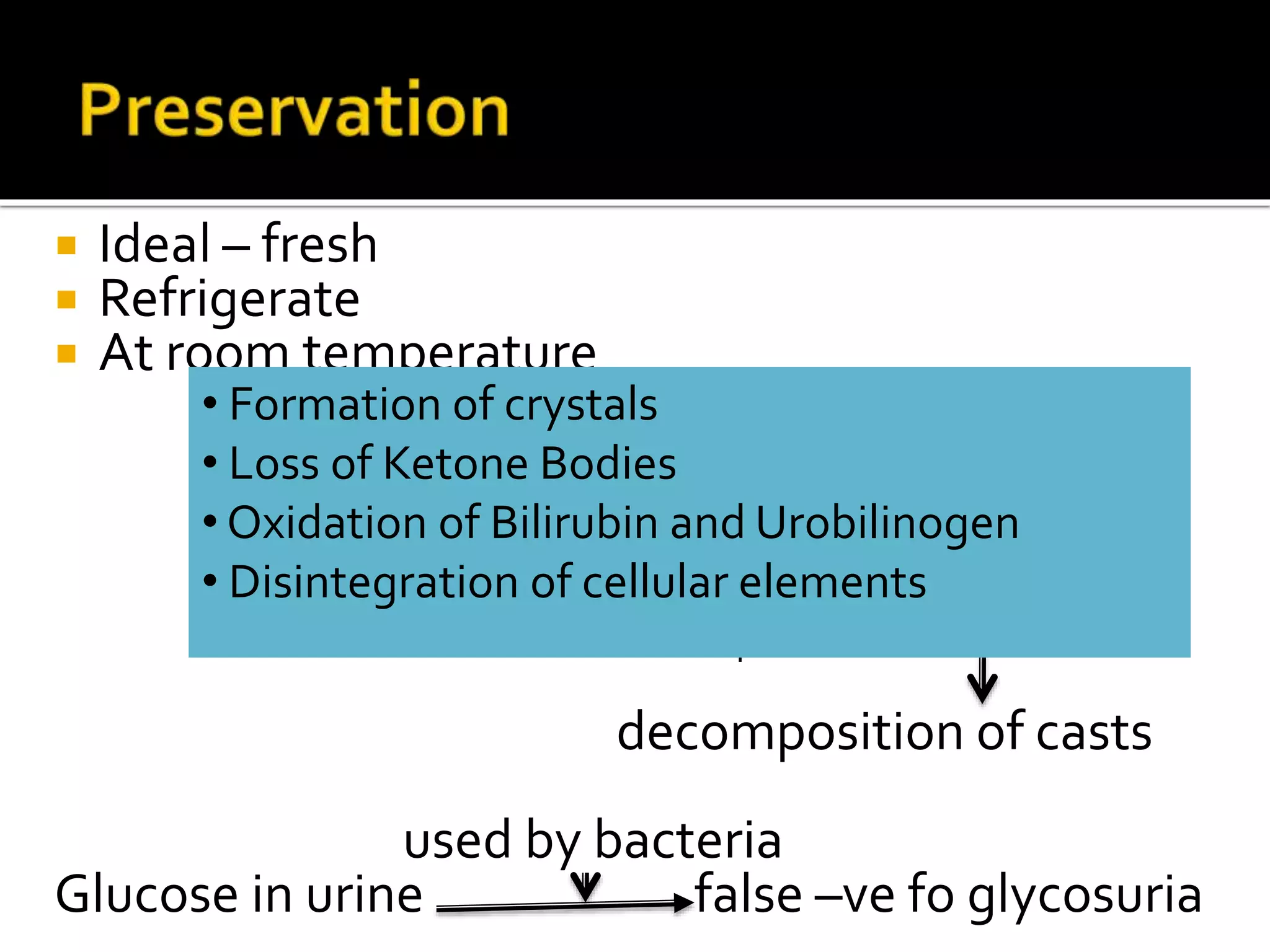
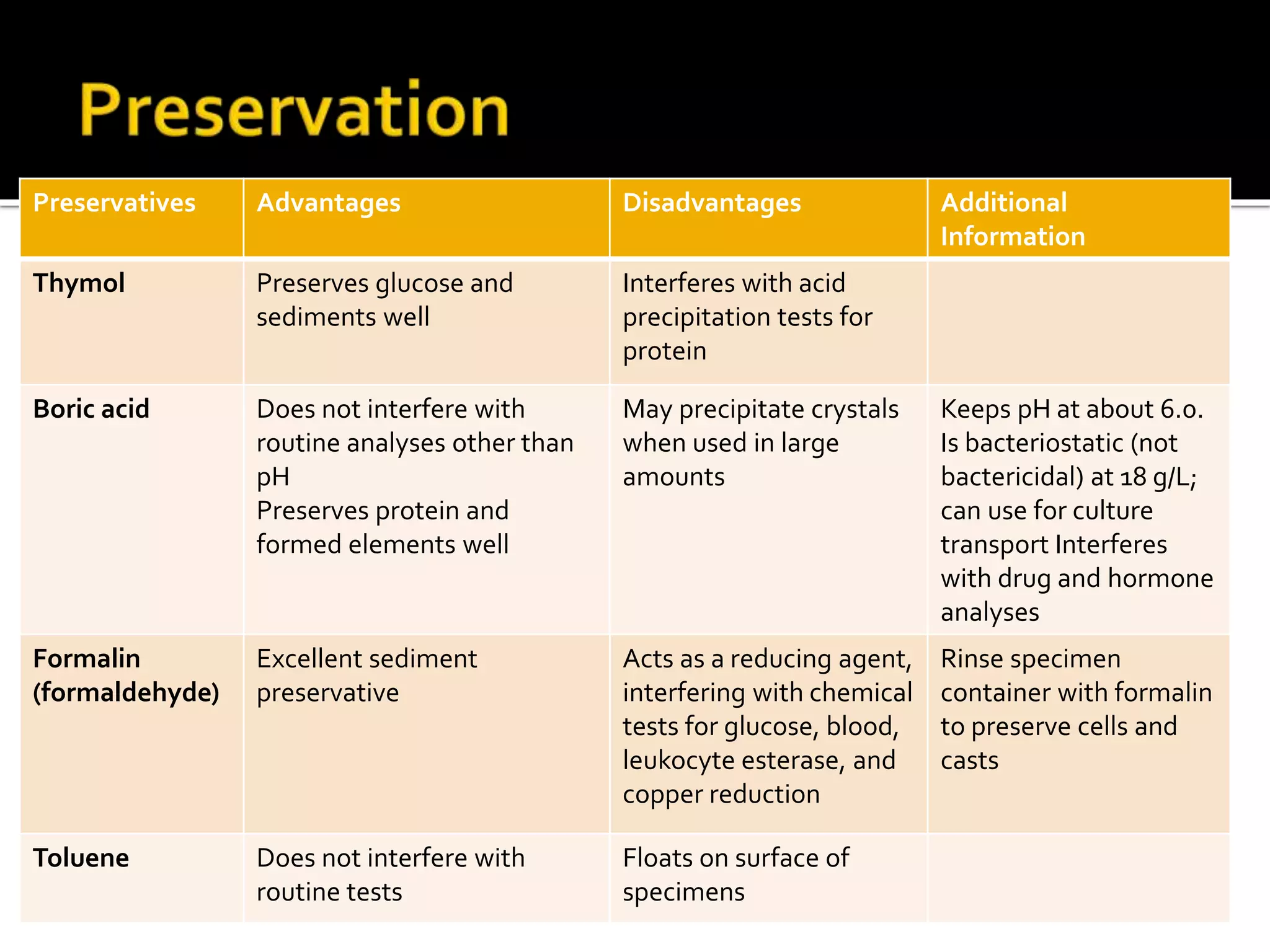
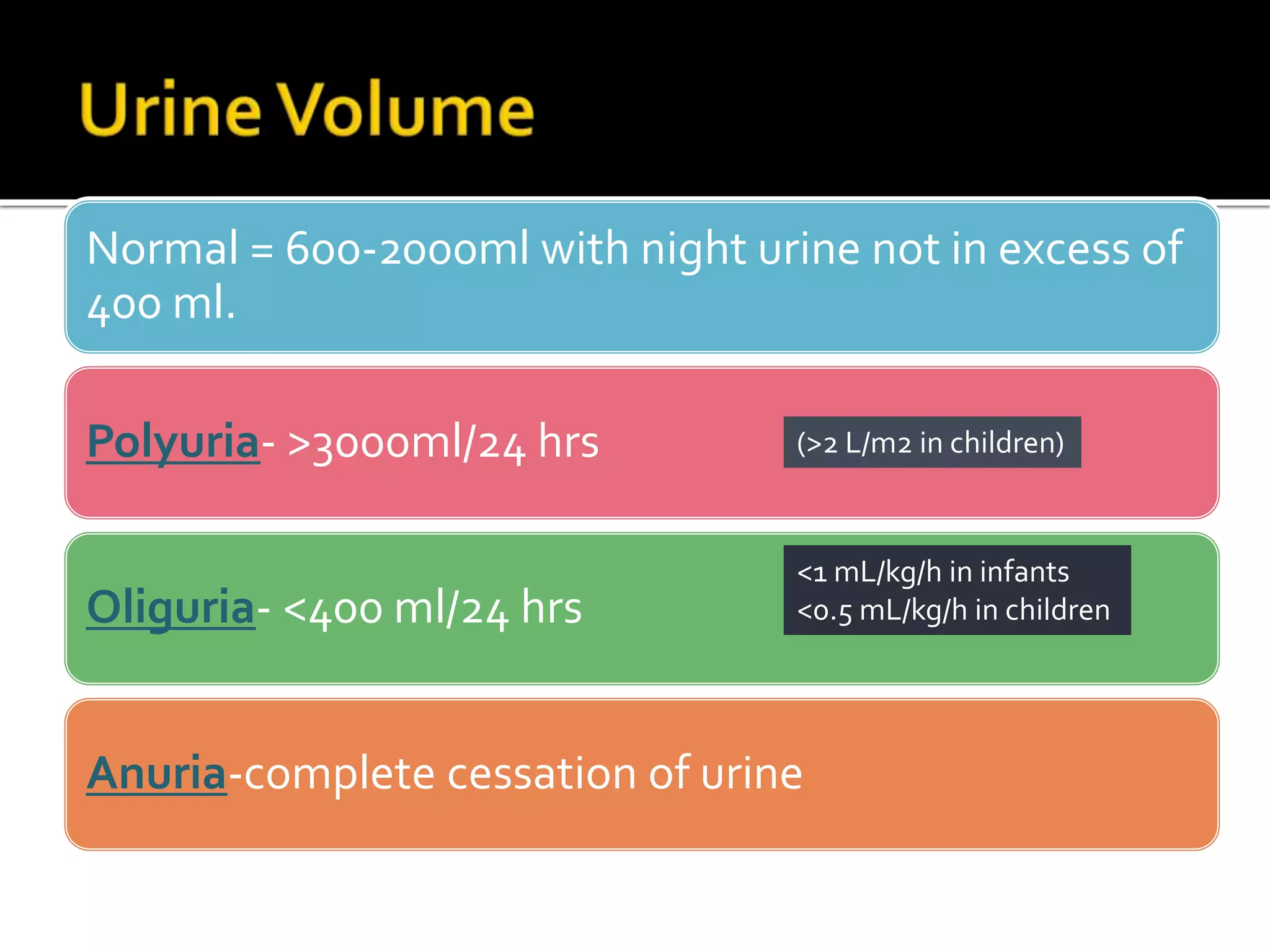
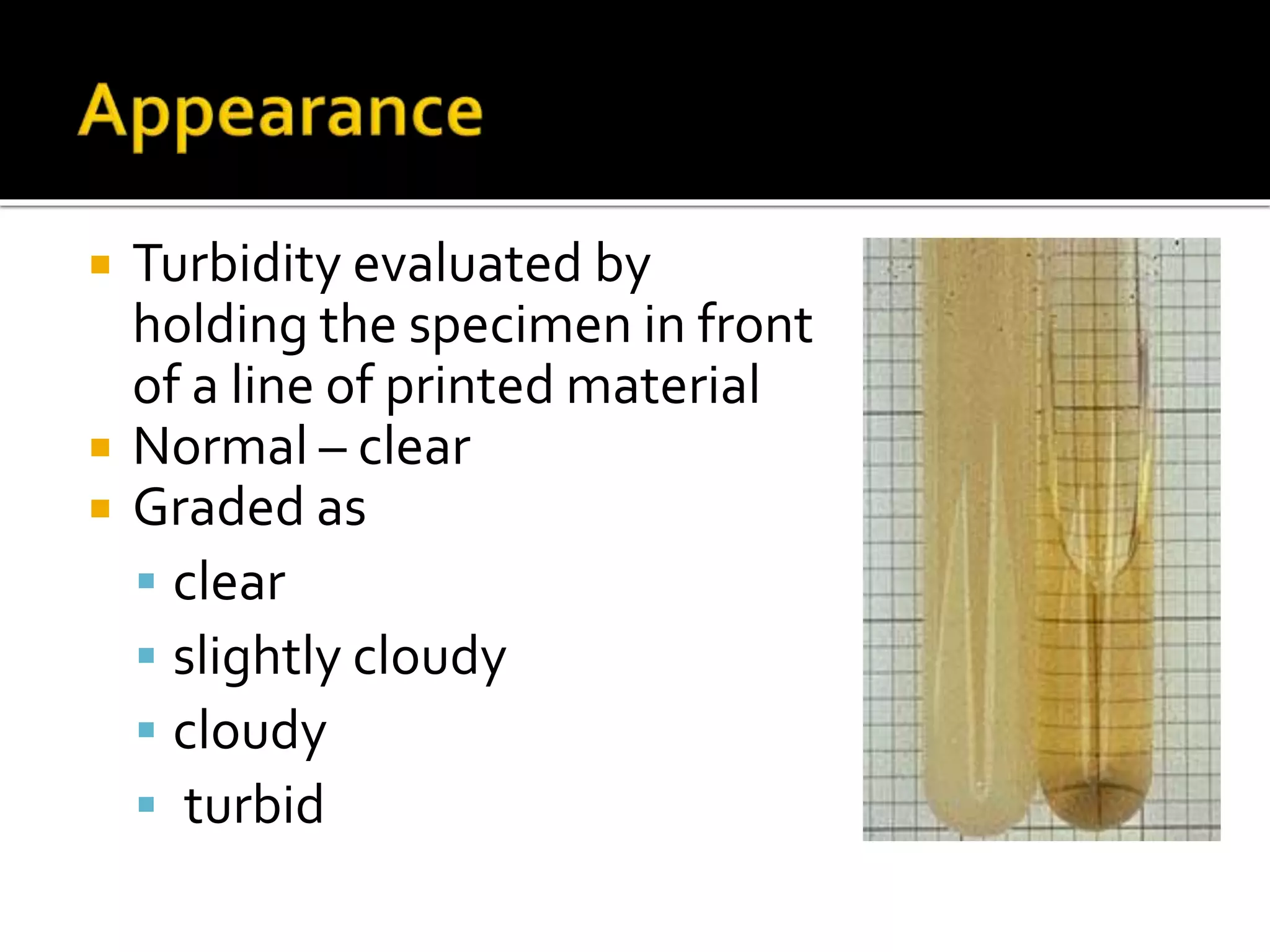
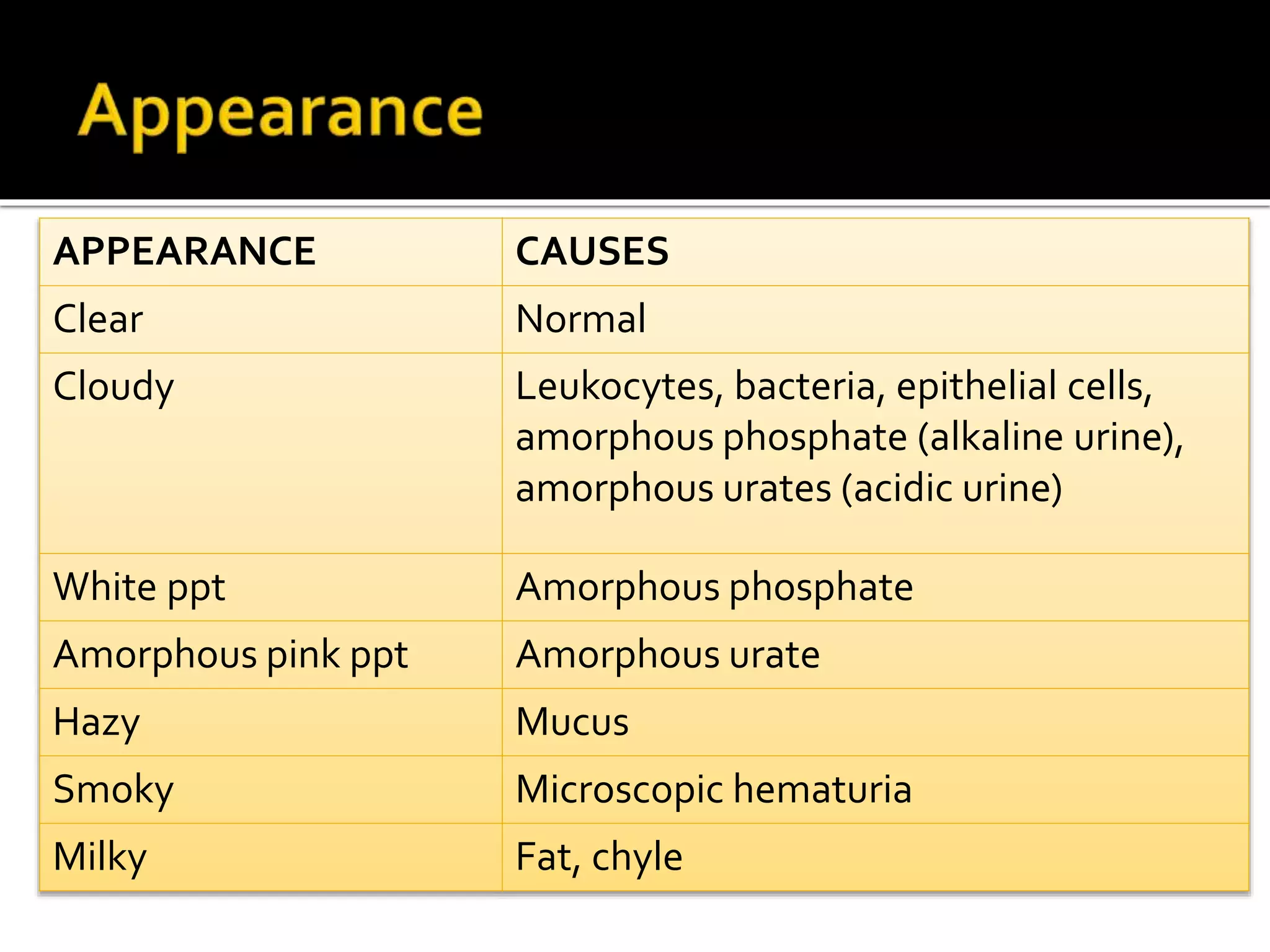
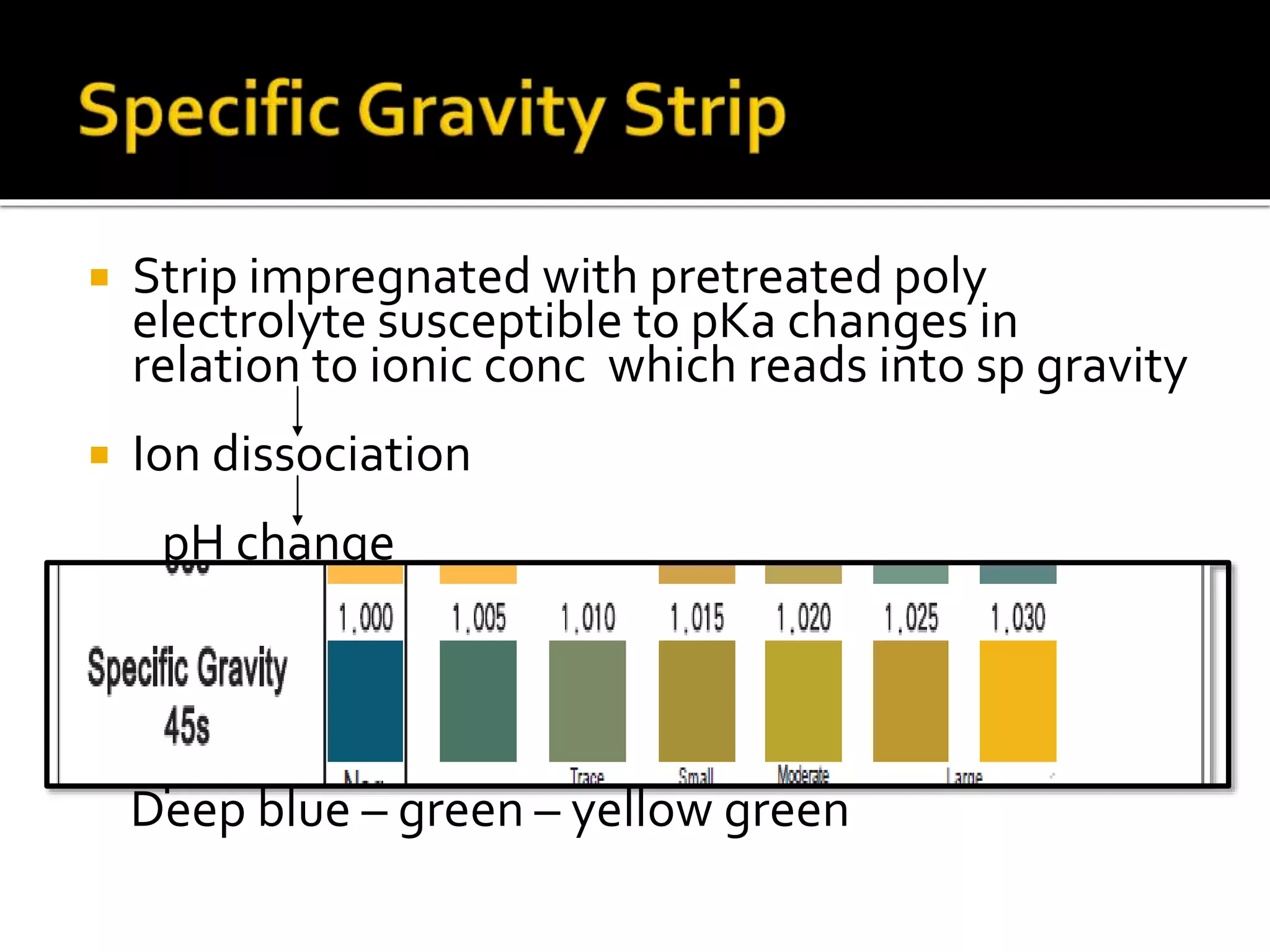
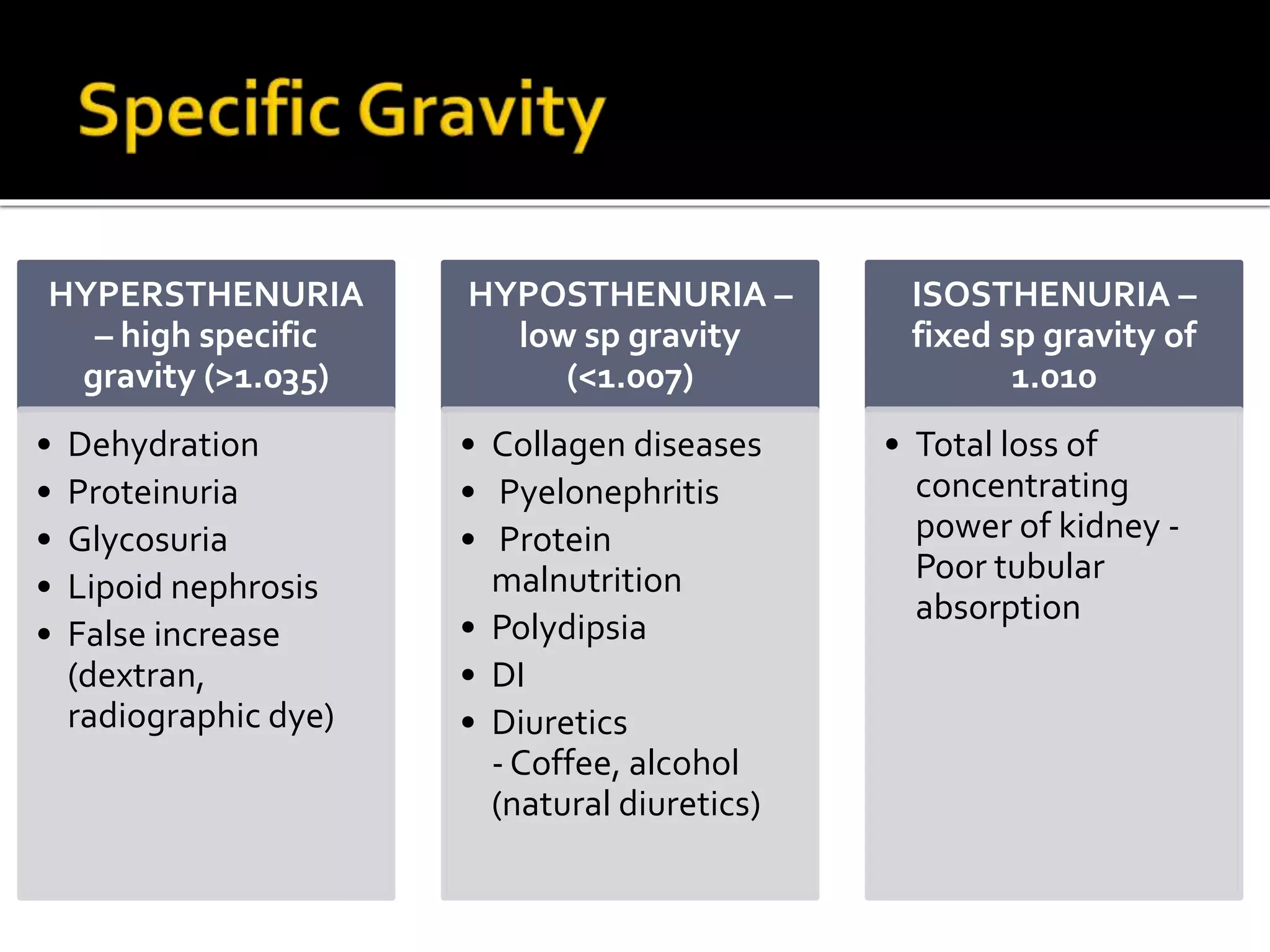
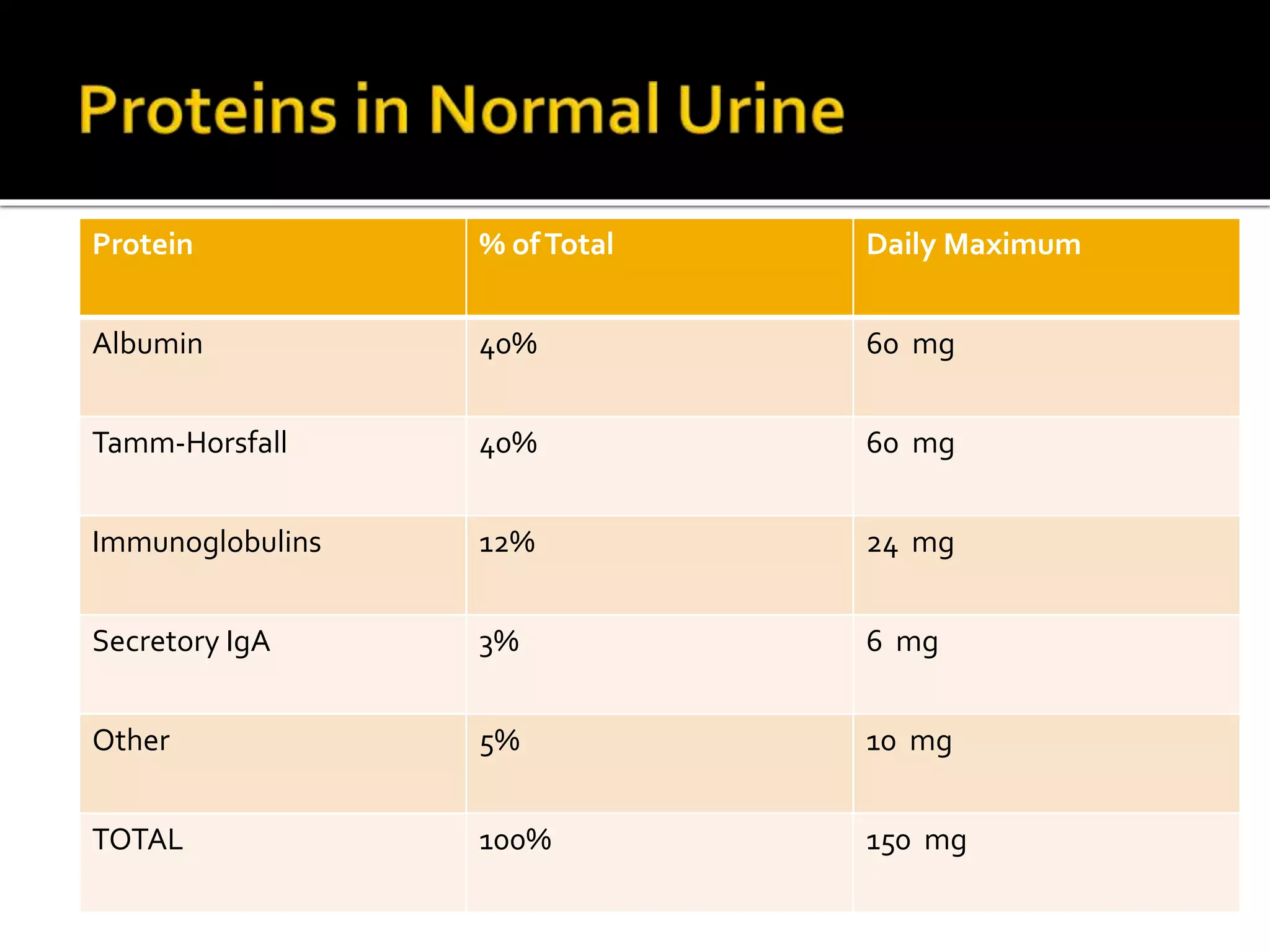
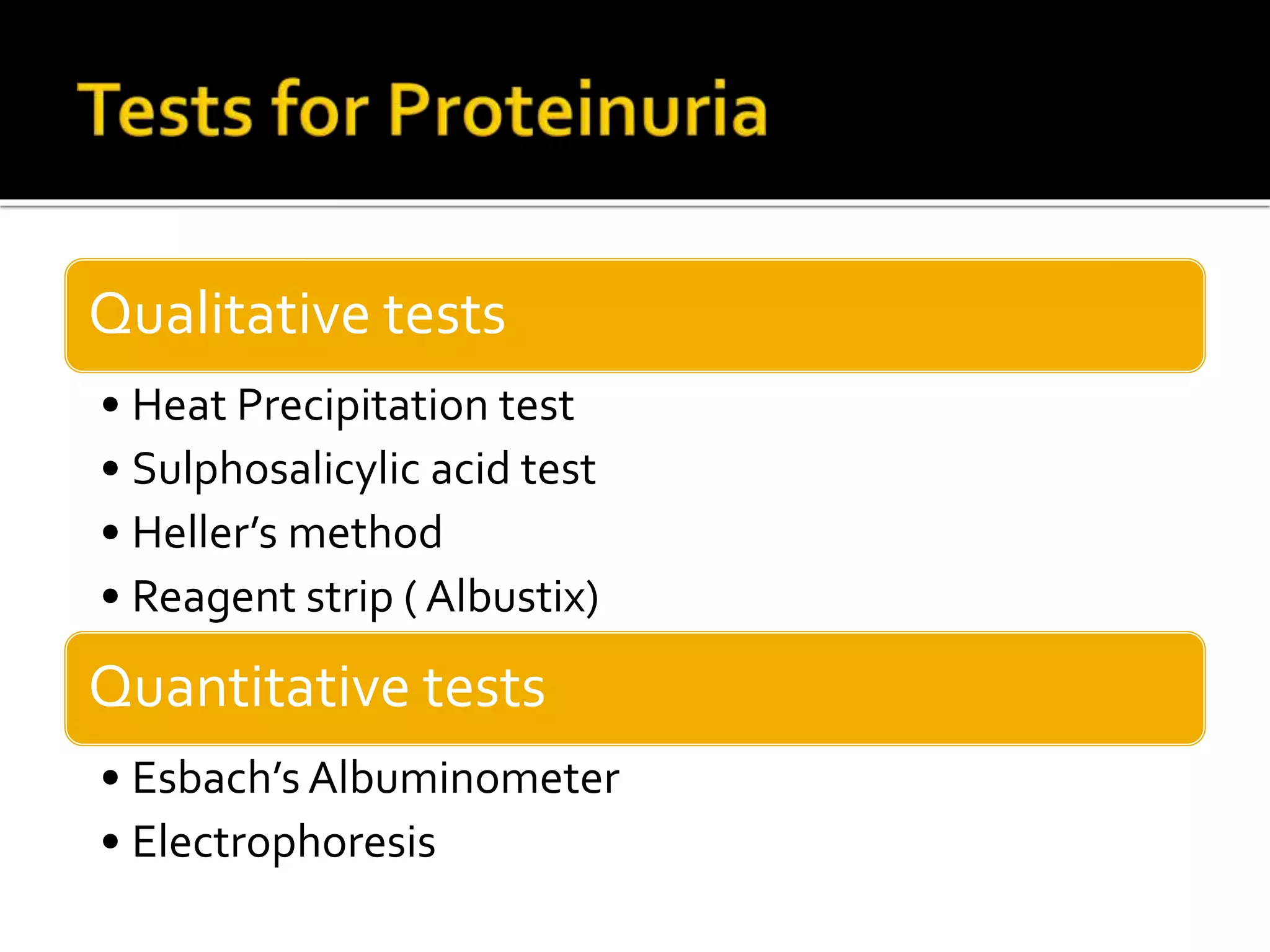
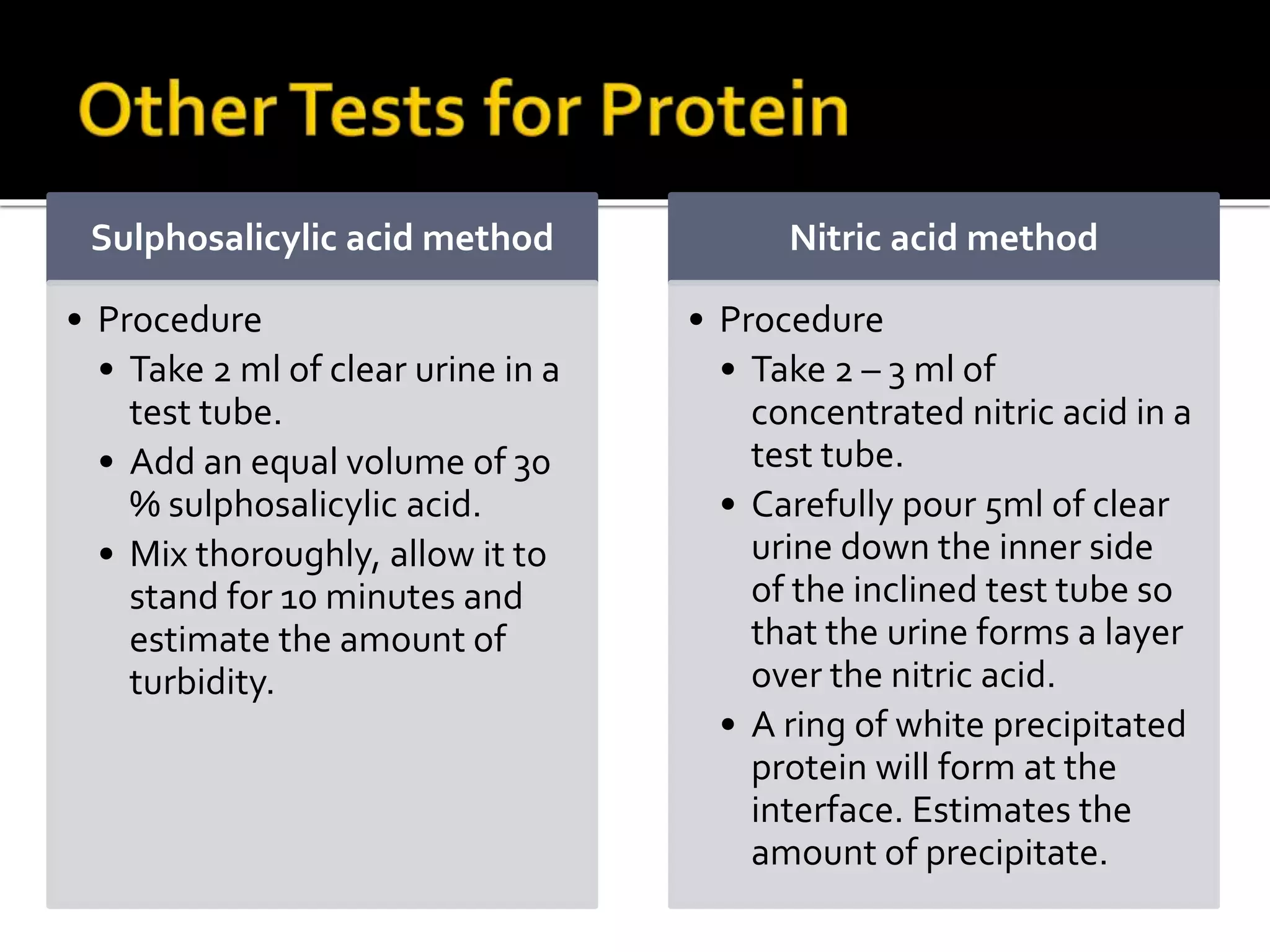
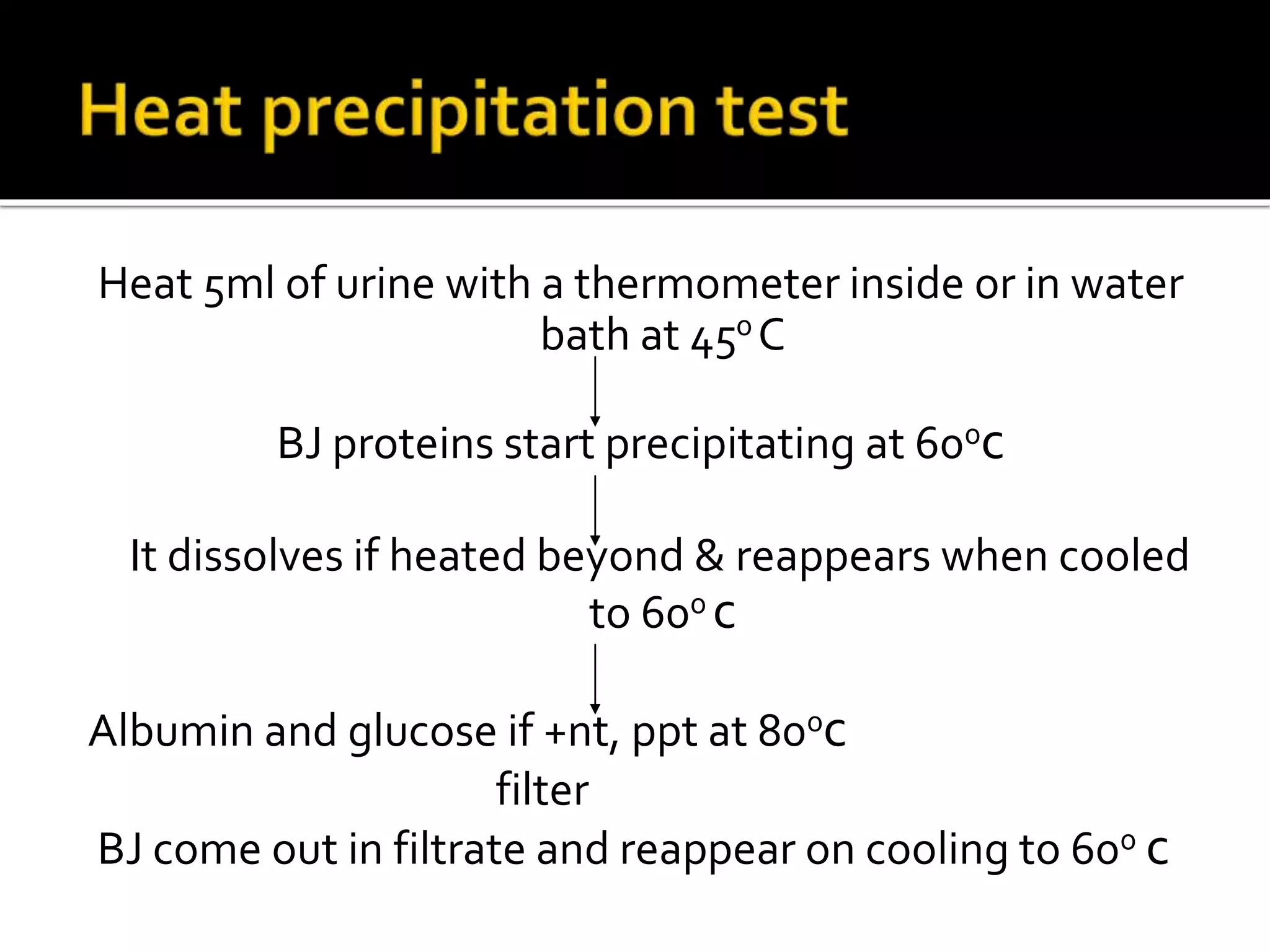
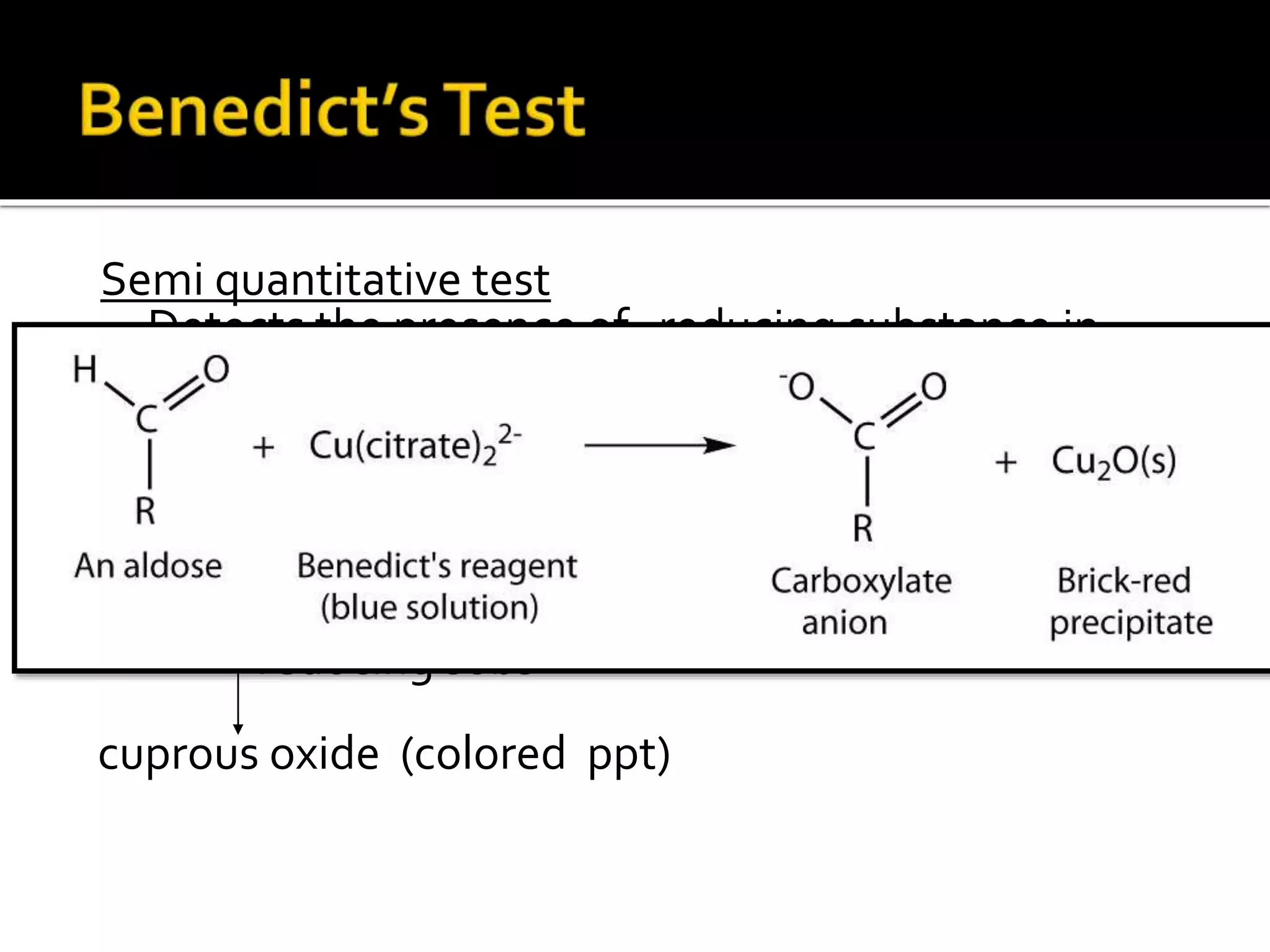
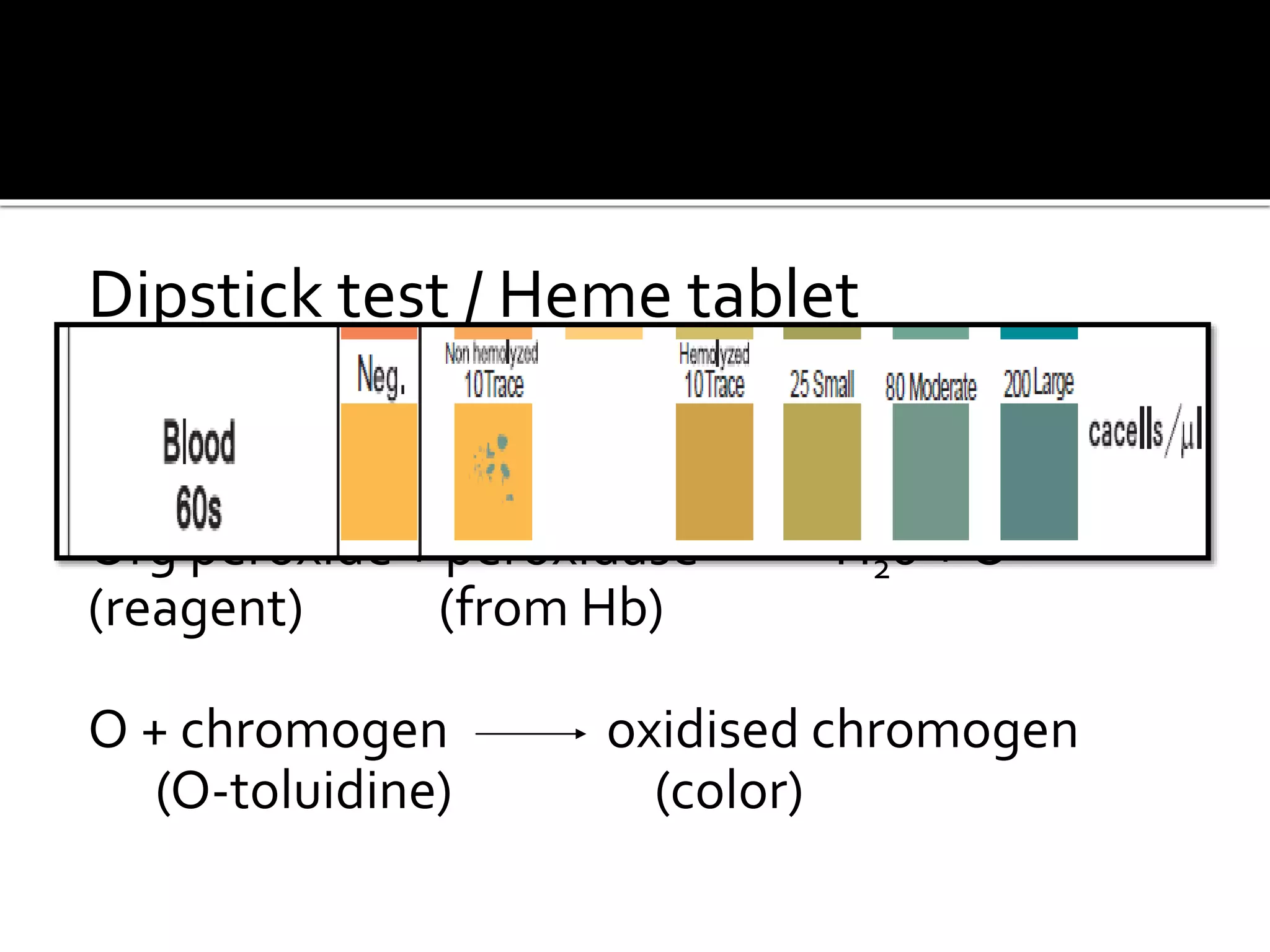
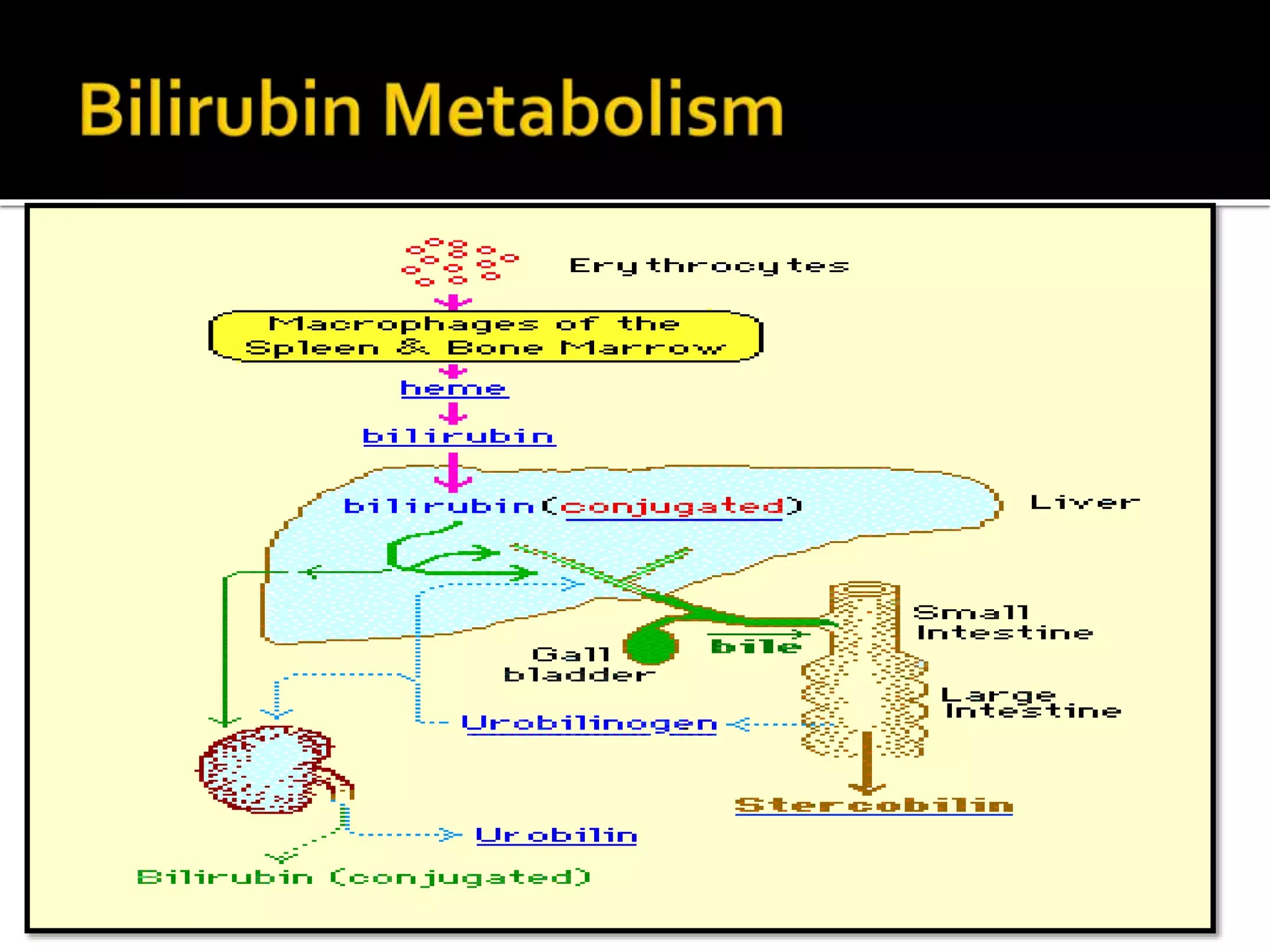
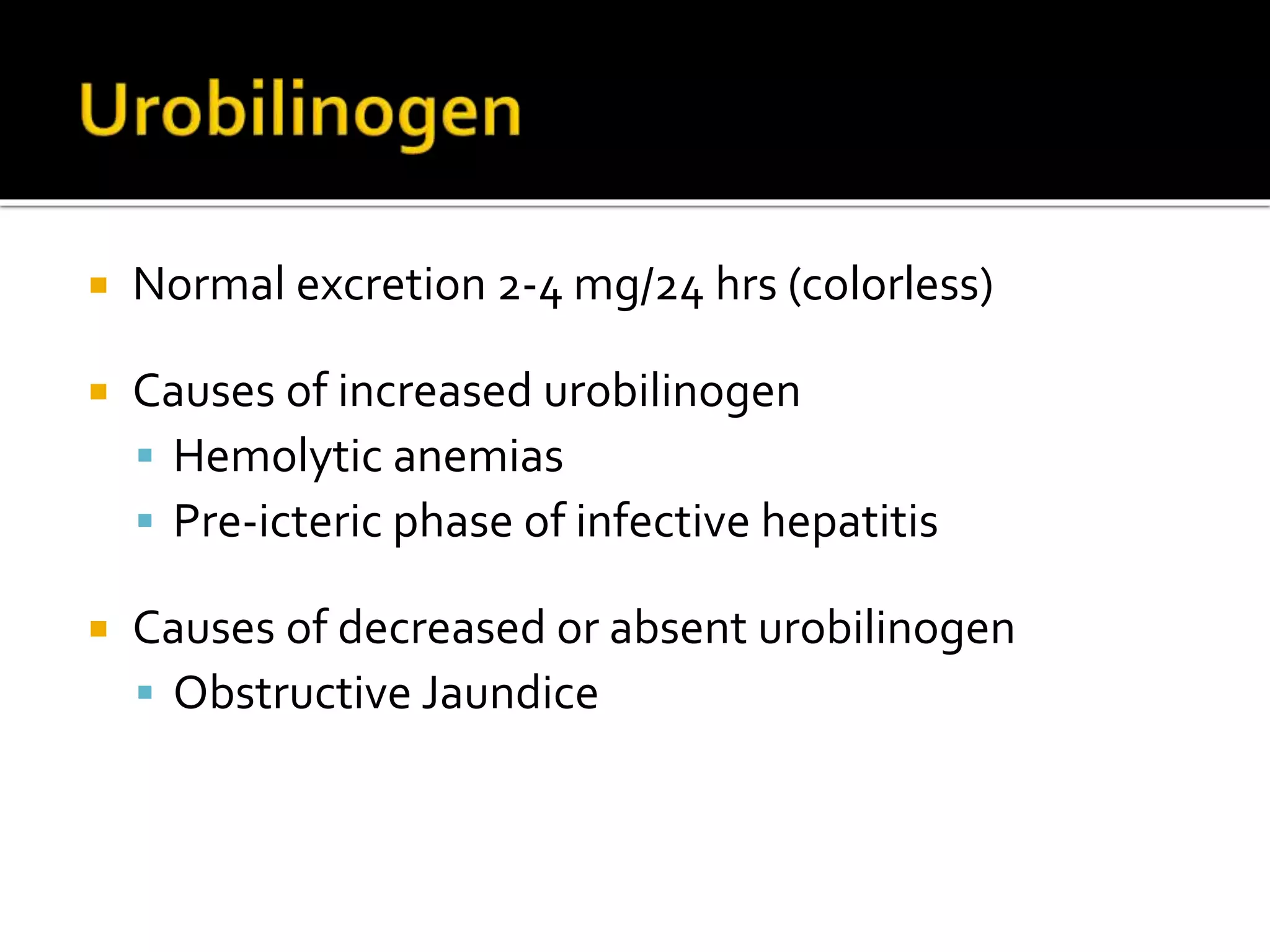
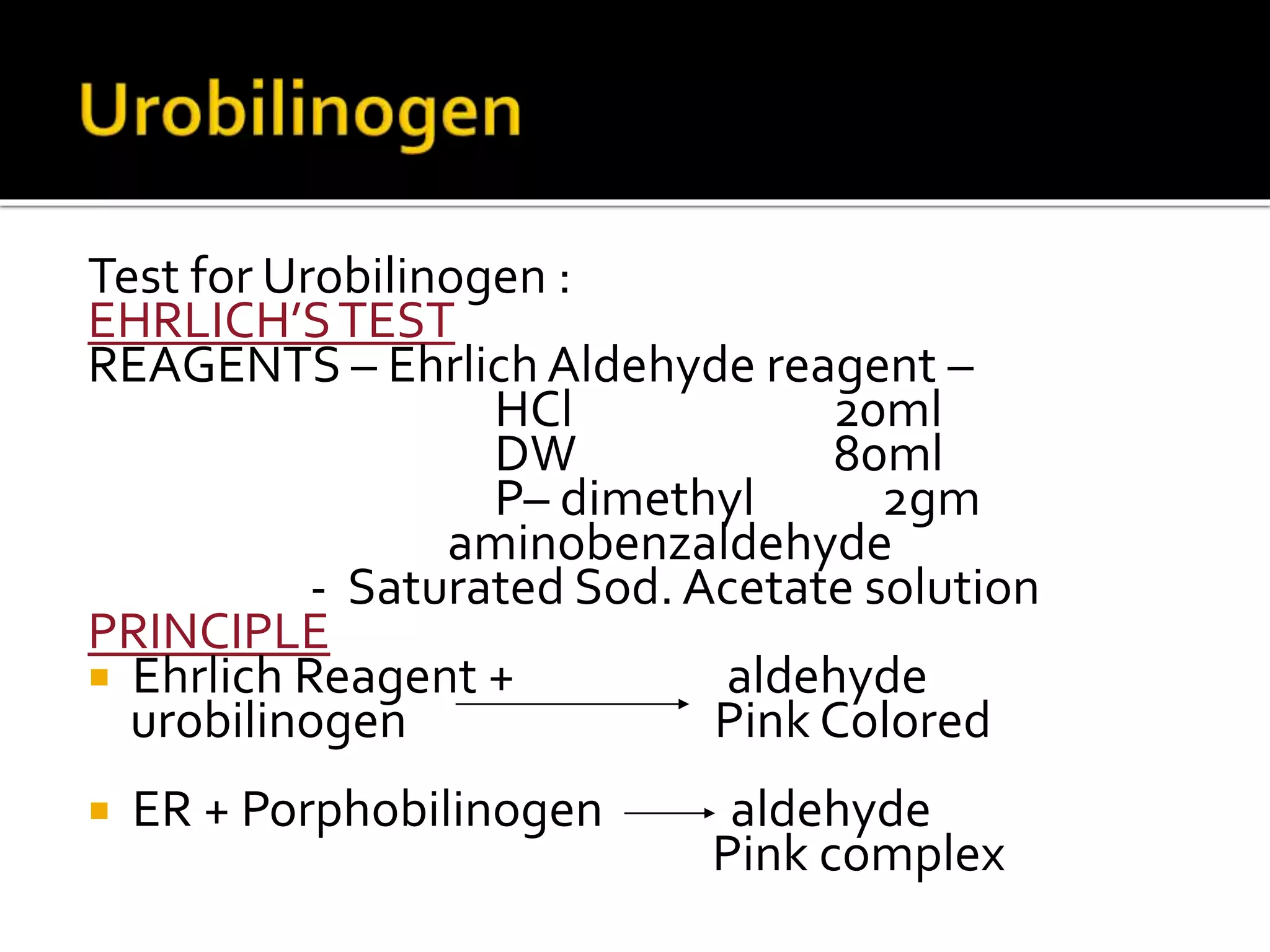
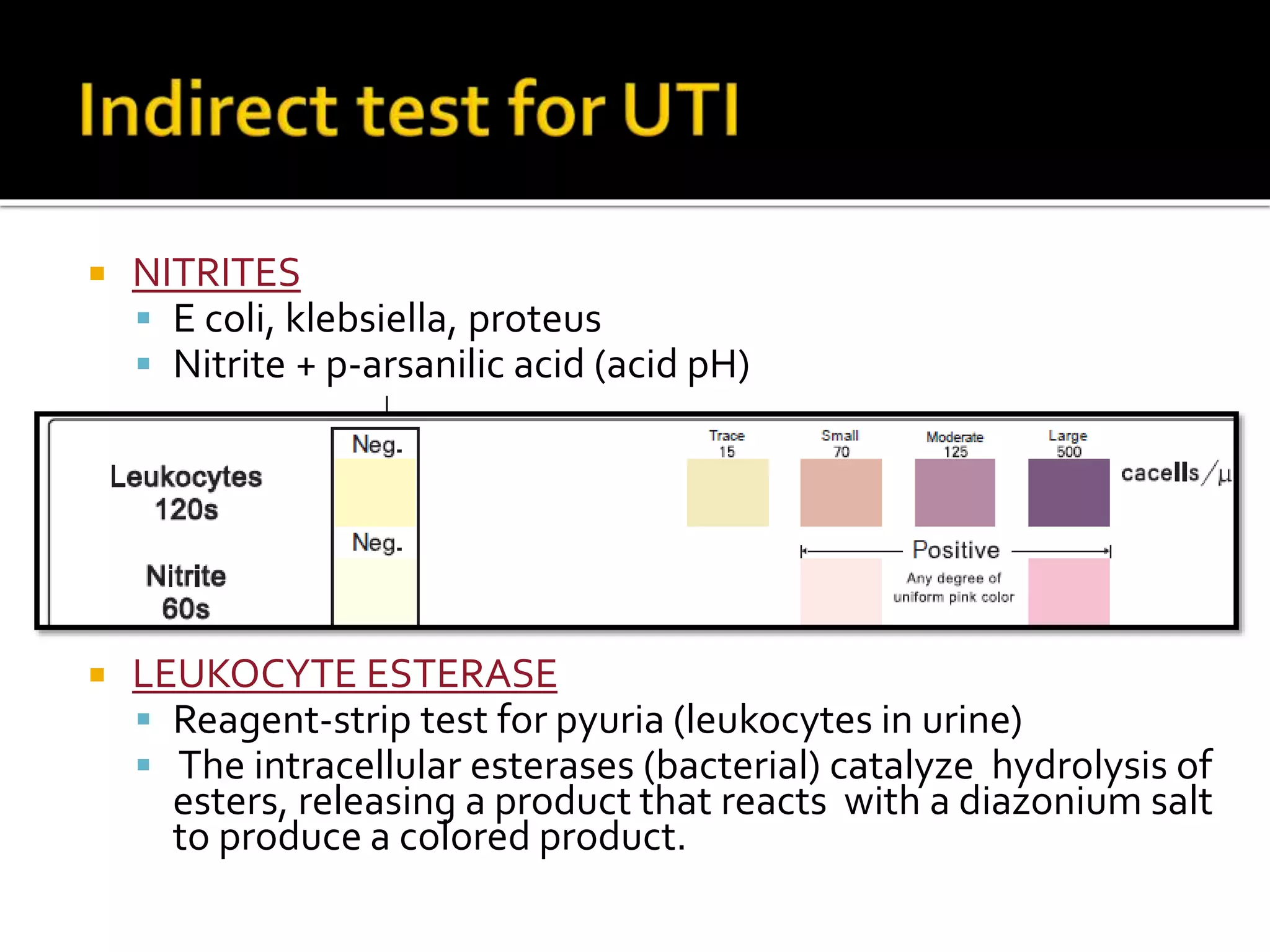
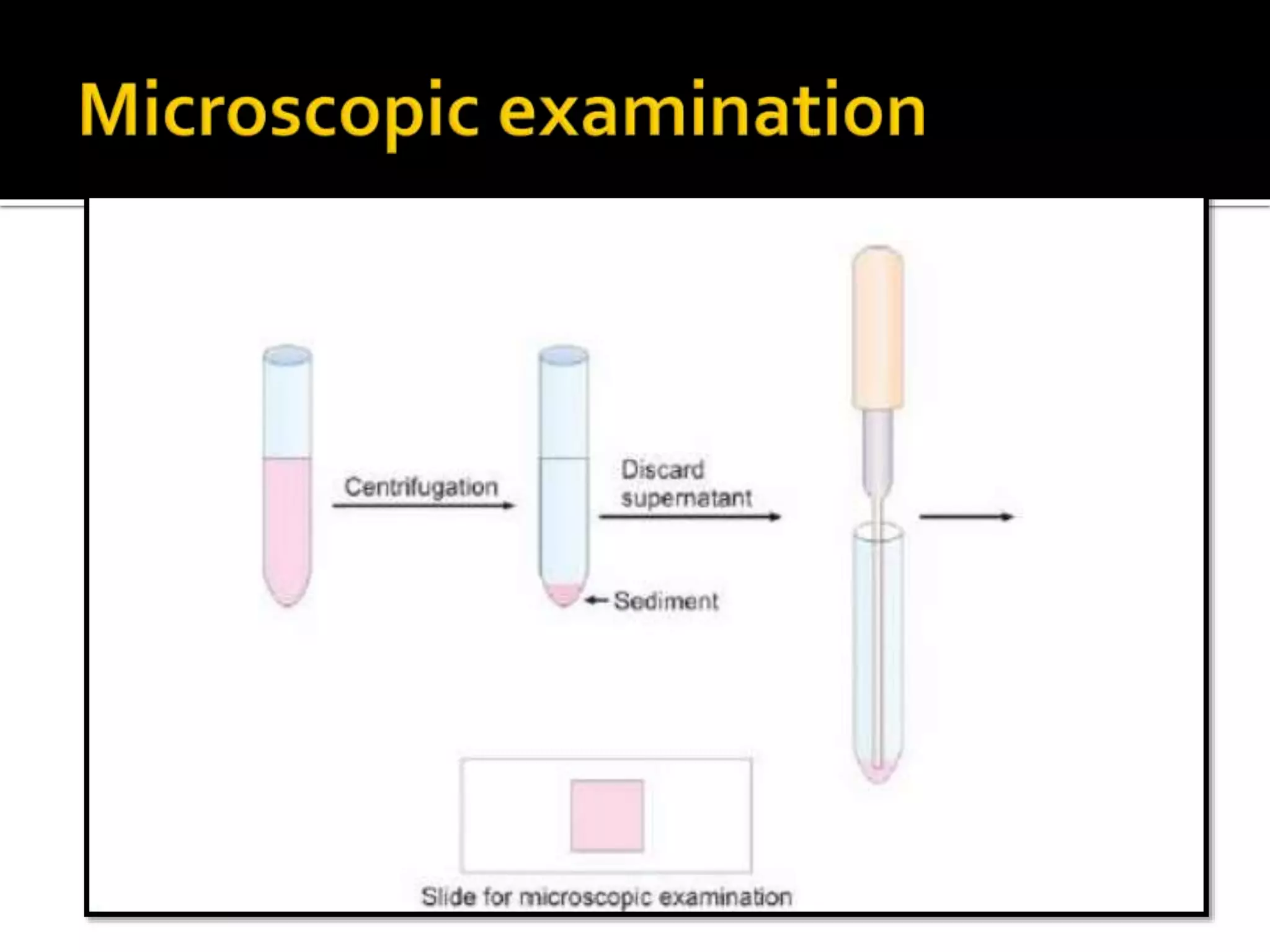
Urine reflects the overall metabolic and kidney functions of the body. It can be used for diagnosis of structural and functional disorders of the urinary system as well as for monitoring diseases and detecting systemic disease processes. Urine can be analyzed for various properties including pH, protein, ketone bodies, blood, and bile salts and bile pigments to provide information about the patient's condition. Various tests exist for qualitative and quantitative analysis of these urine components.