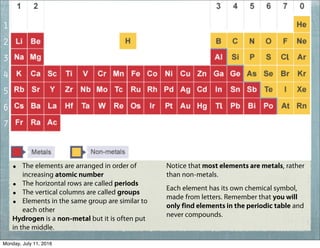
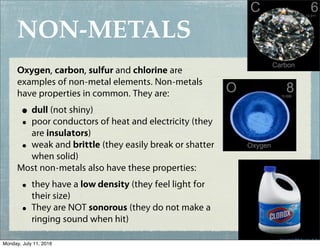
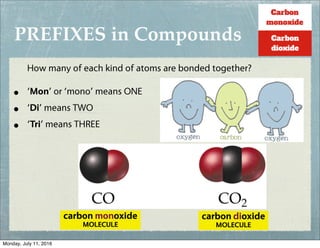
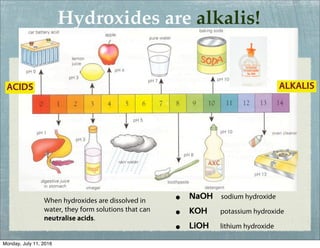
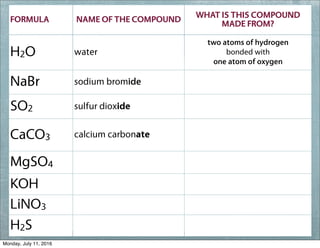
This document provides an overview of elements, compounds, and chemical formulas. It discusses how elements are arranged on the periodic table and classified as metals and non-metals. Metals are described as shiny, good conductors or heat/electricity, and malleable, while non-metals are dull, poor conductors, and brittle. Compounds are formed when different types of atoms bond together into molecules. Chemical formulas represent the elements that make up compounds and use prefixes to indicate the number of each atom. Common compounds like water, sodium chloride, and calcium carbonate are presented along with their formulas.