Embed presentation
Downloaded 137 times
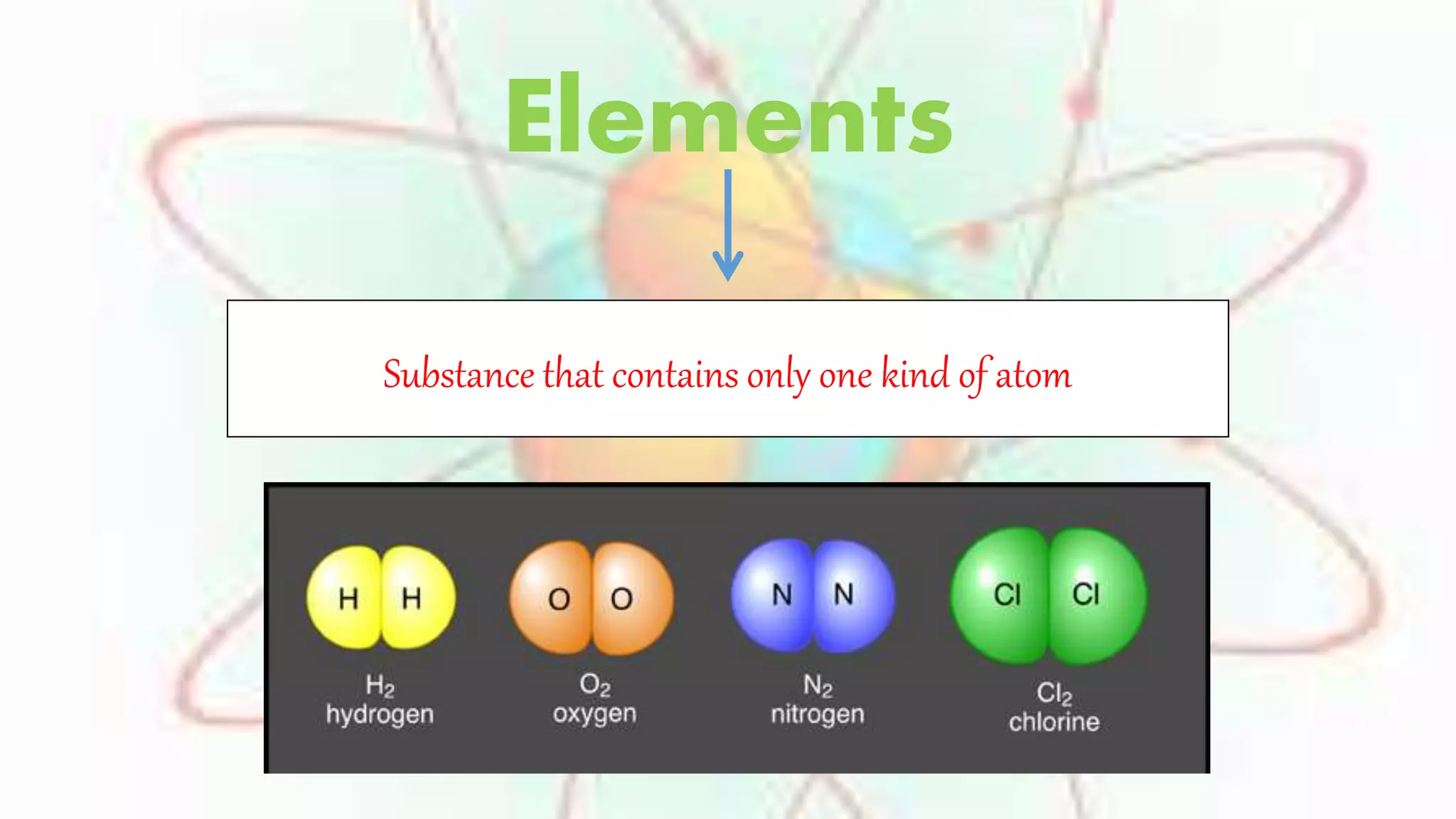
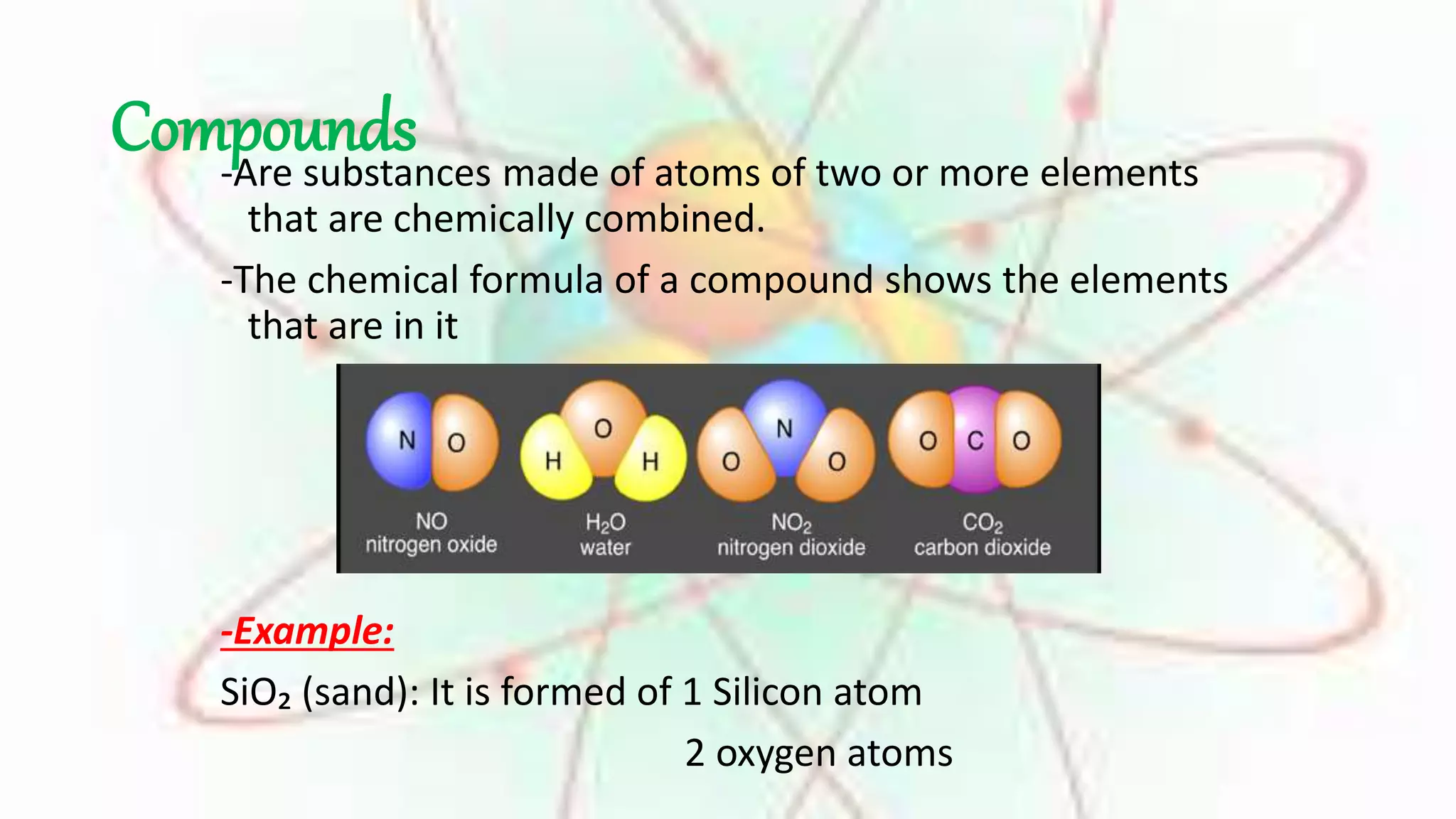


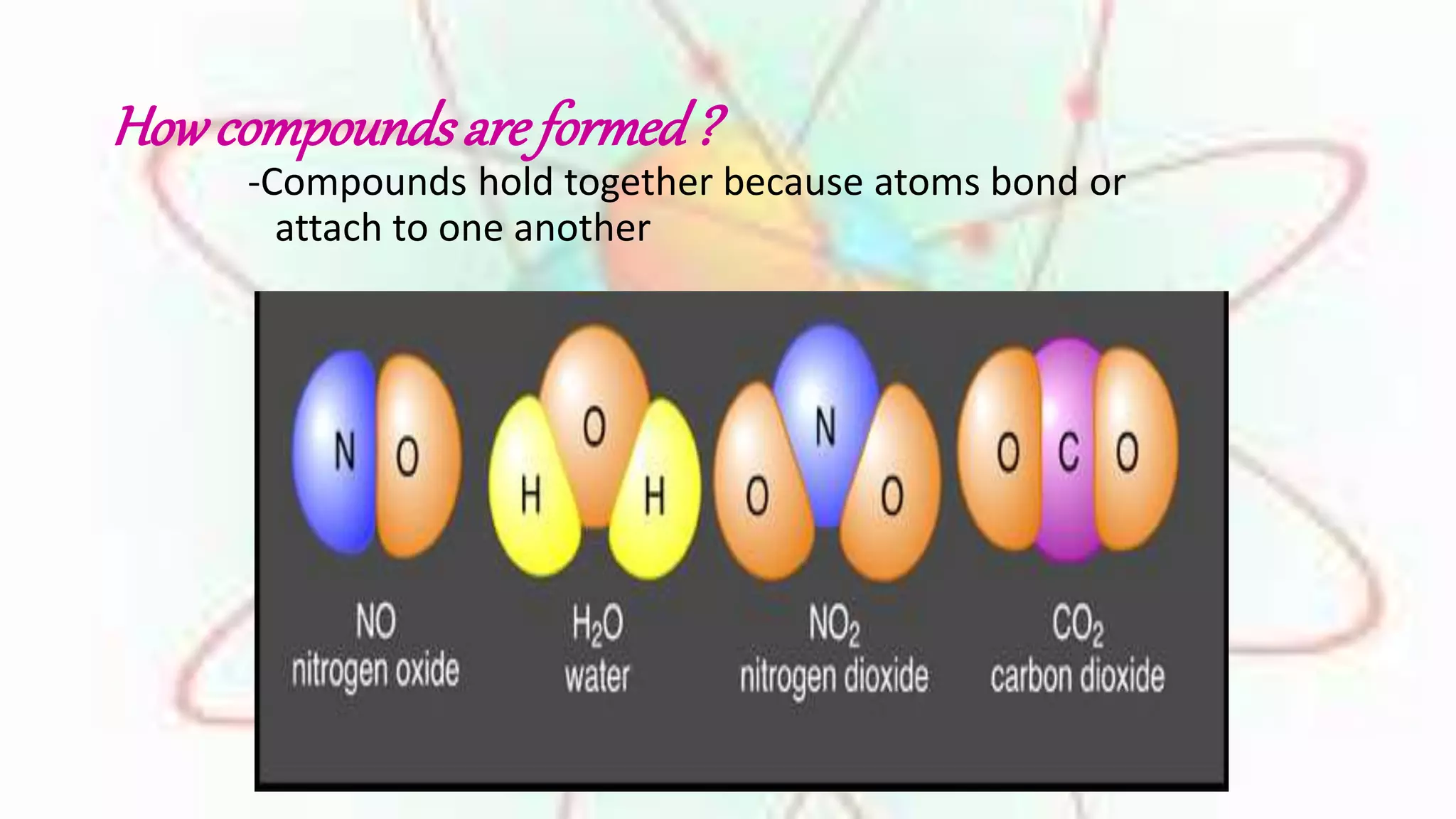
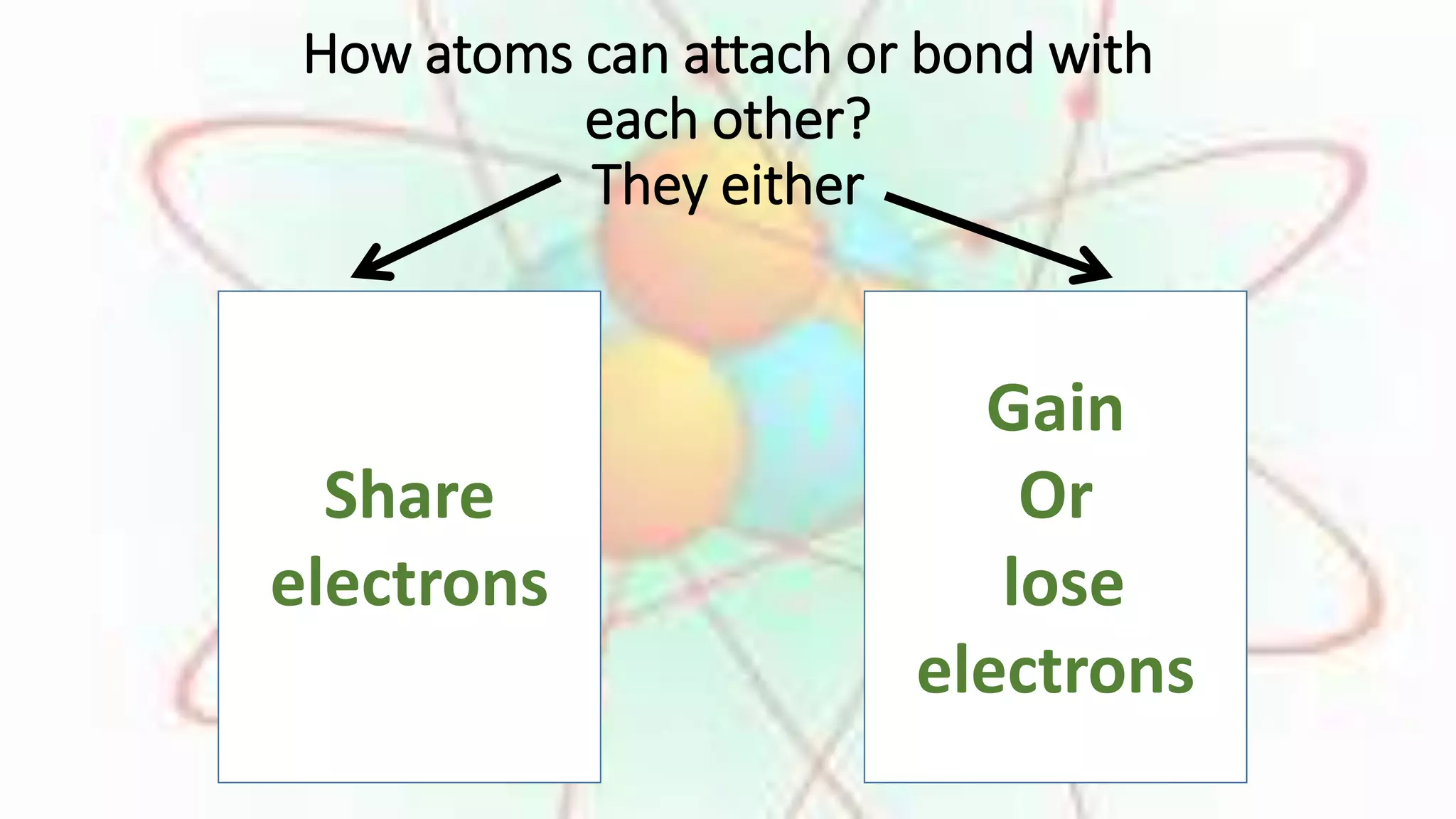
Elements are substances made of only one type of atom, while compounds are made of two or more elements that are chemically bonded together. The chemical formula of a compound shows which elements it contains. Compounds have different properties than their constituent elements. Common table salt, or NaCl, is a compound formed from the elements sodium and chlorine bonding together, even though on their own sodium is a metal and chlorine is a gas. Compounds form when atoms bond by sharing, gaining or losing electrons.





