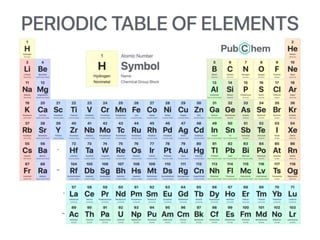
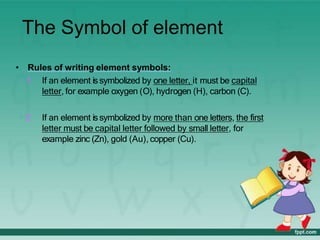
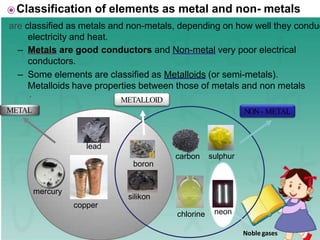
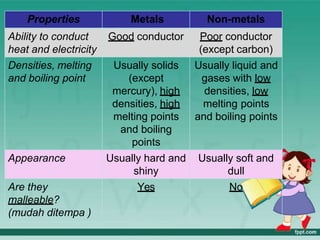
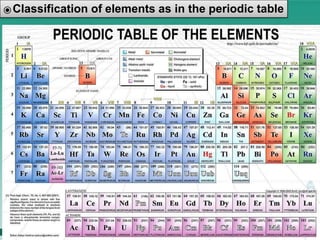
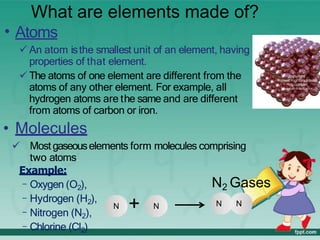
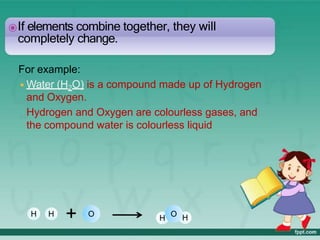
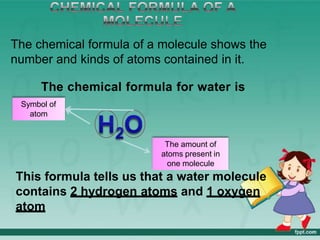
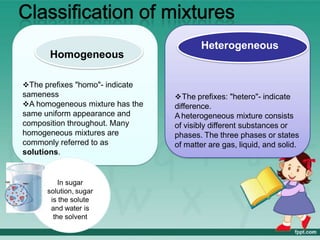
This document defines elements, compounds, and mixtures. It provides examples of common elements like hydrogen, oxygen, and carbon. Elements are made of atoms and cannot be broken down further. Compounds are formed when elements chemically combine in molecules. Compounds have different properties than their constituent elements. Mixtures contain elements or compounds mixed together but not chemically combined. Mixtures can be either homogeneous, with a uniform composition, or heterogeneous, containing distinct substances or phases.