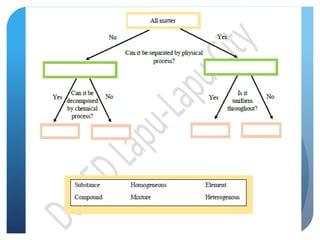
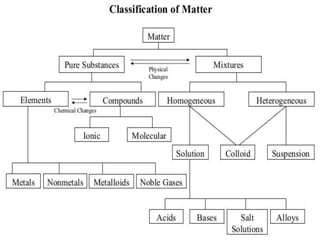
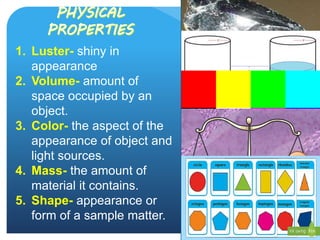
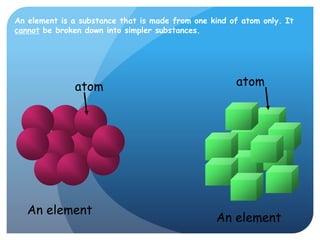
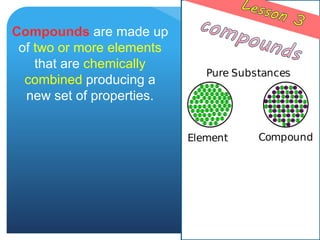
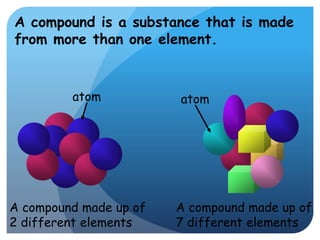
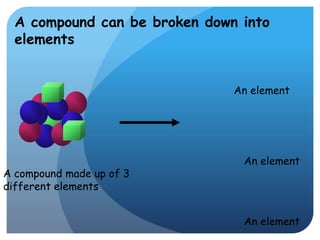
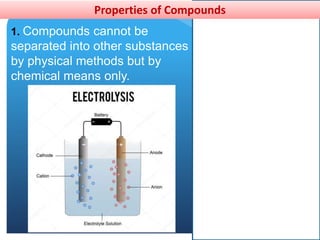
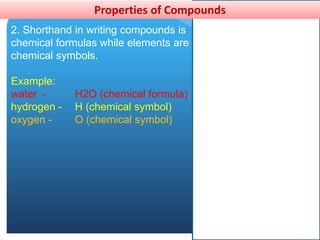
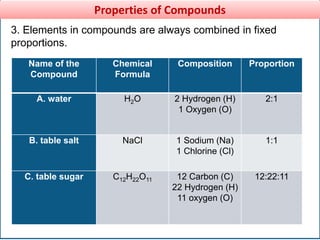
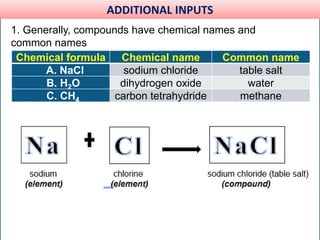
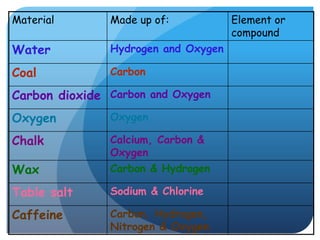
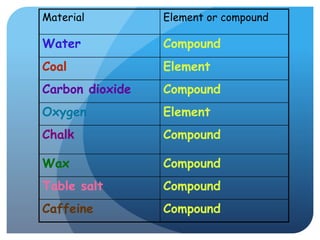
This document provides information about elements and compounds for a 7th grade science class. It defines an element as a pure substance made of only one type of atom that cannot be separated into simpler substances. An element can combine with other elements to form compounds. A compound is made of two or more elements chemically bonded together to form a new substance with unique properties. The document lists examples of elements like copper and carbon, and compounds like water and table salt. It describes physical and chemical properties of elements and characteristics of compounds.