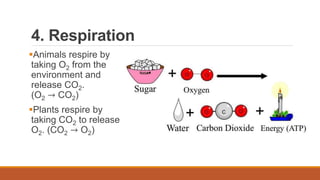
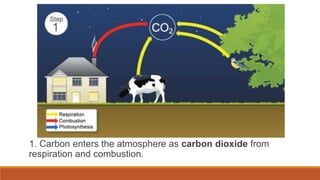
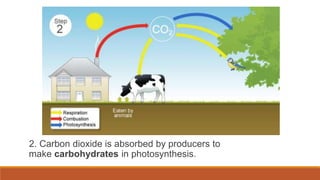
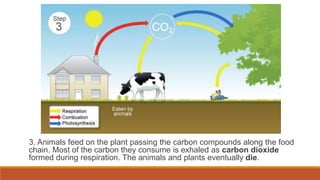
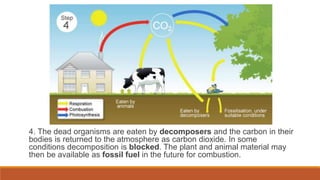
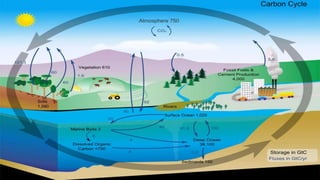
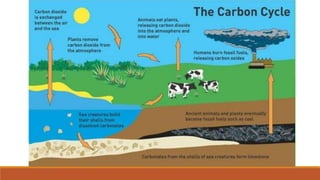
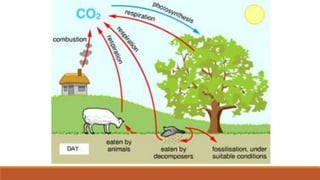
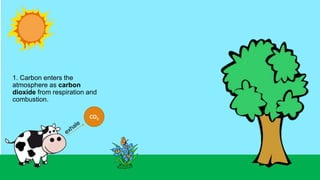
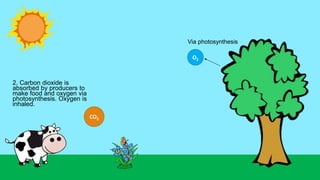
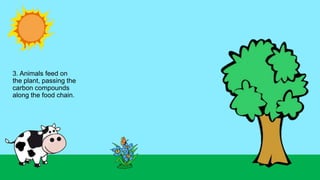
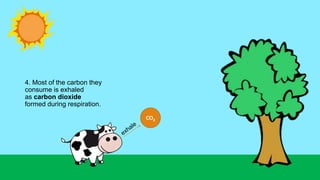
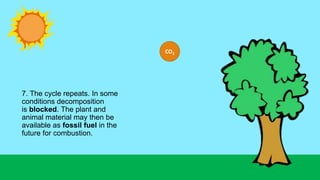
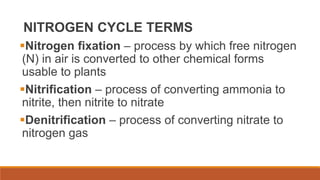
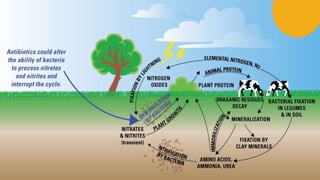
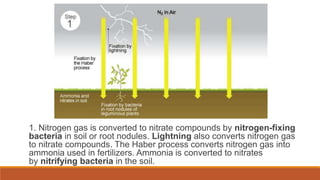
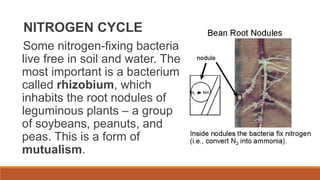
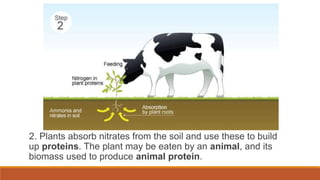
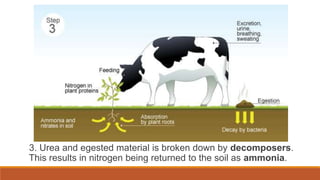
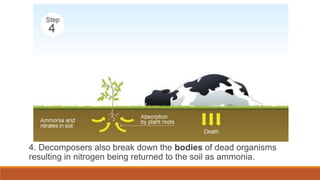
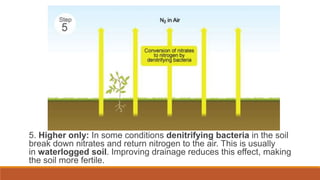
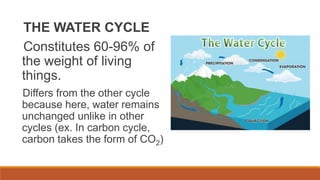
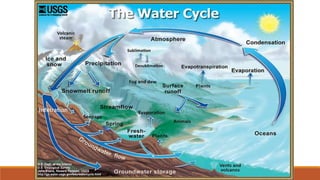
This document outlines nutrient cycles, including the carbon, nitrogen, and water cycles, highlighting the processes of nutrient recycling essential for living organisms. It details how nutrients like carbon and nitrogen are transformed and used by plants and animals, alongside the different stages of each cycle, such as decomposition, nitrification, and evaporation. Additionally, it explores the impact of human activities like deforestation and fossil fuel burning on these cycles and discusses the importance of various forms of nitrogen fixation.