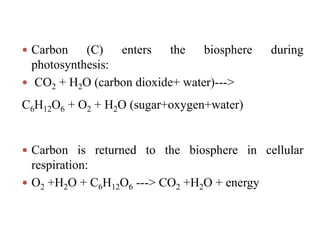
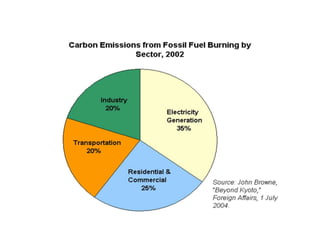
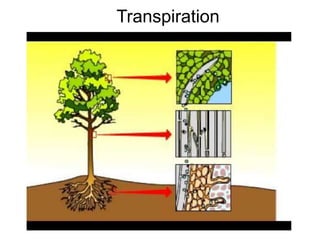
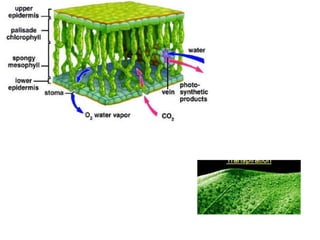
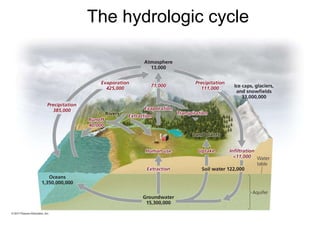
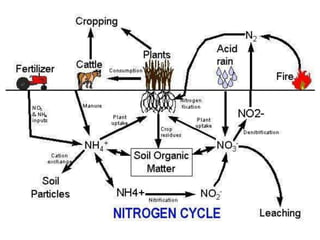
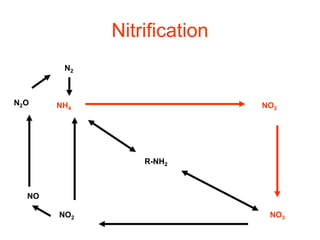
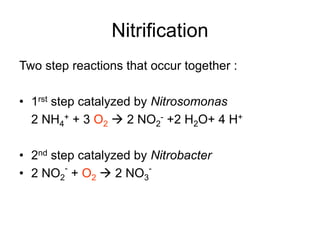
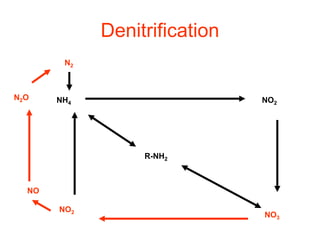
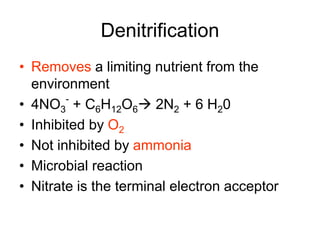
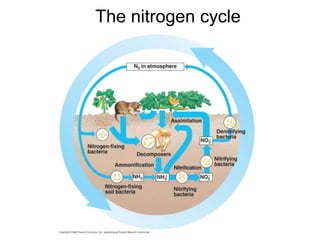
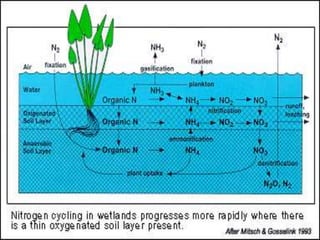
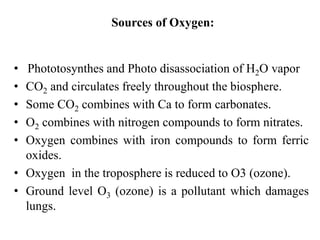
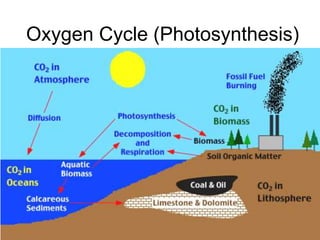
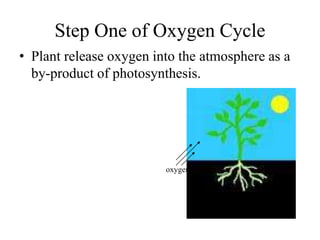
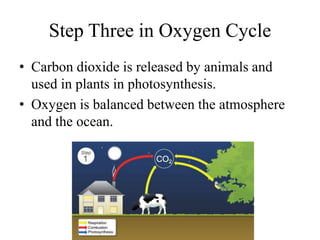
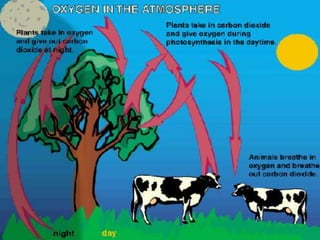
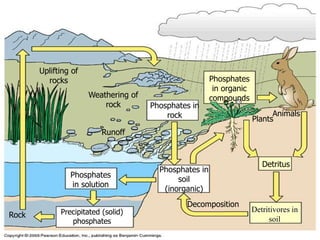
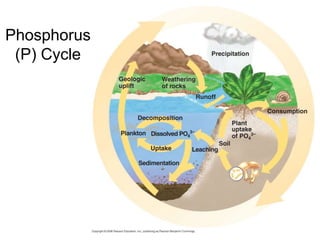
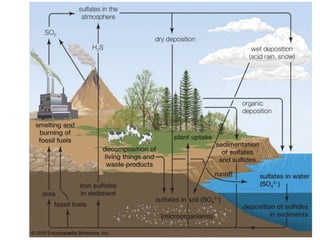
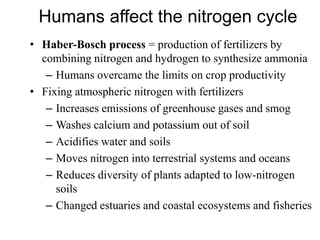
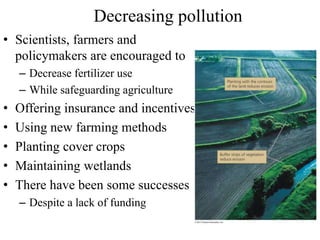
The document summarizes several biogeochemical cycles including the water, carbon, nitrogen, phosphorus, and sulfur cycles. It describes how each element moves through different reservoirs in the Earth's systems and the processes involved. It also discusses how human activities like burning fossil fuels, cutting down forests, agricultural practices, and mining can impact these natural cycles.