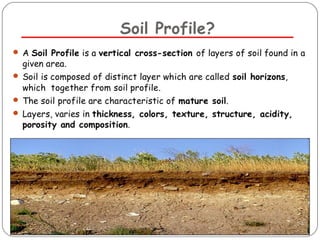
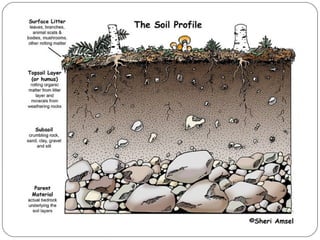
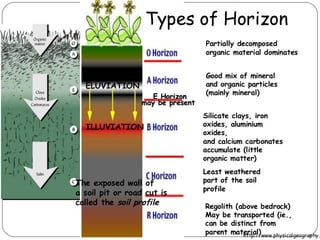
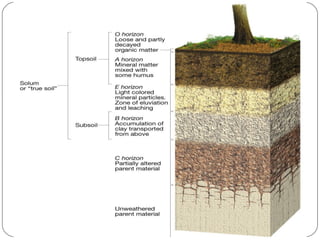
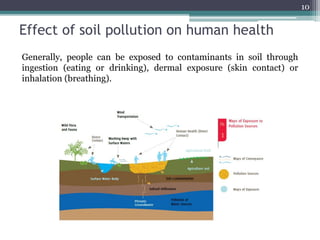
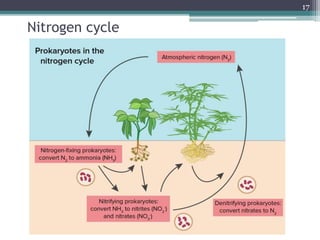
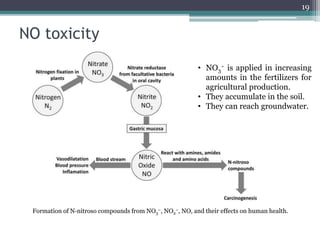
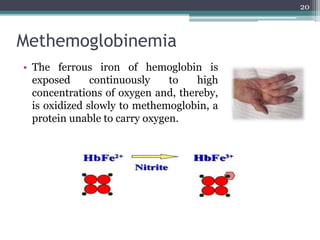
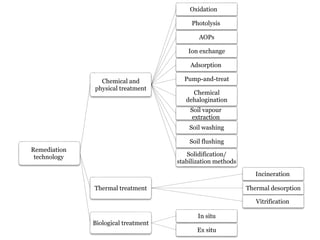
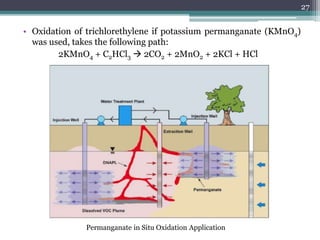
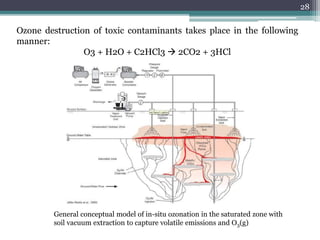
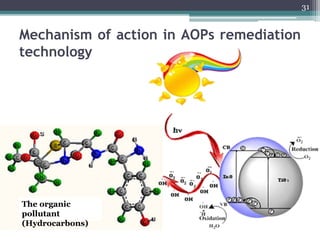
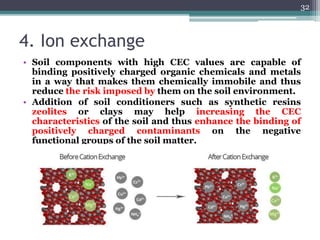
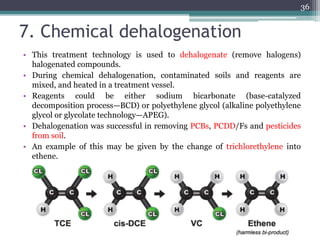
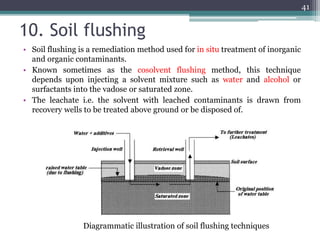
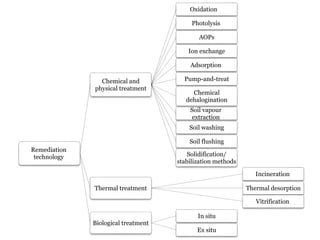
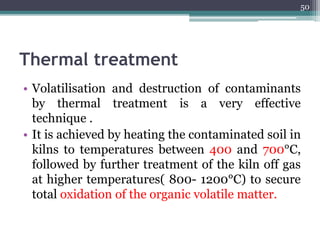
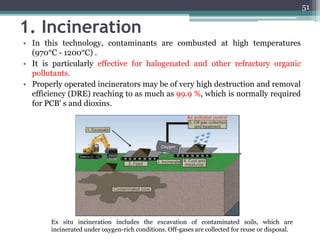
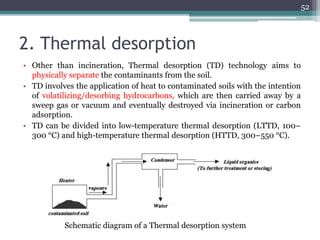
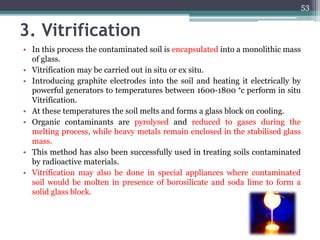
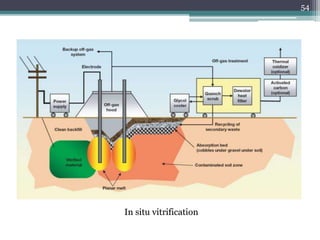
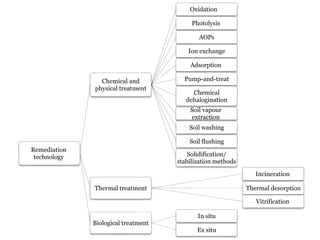
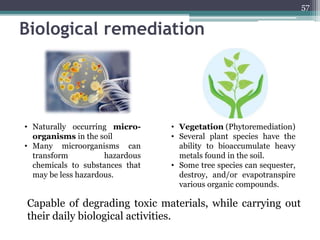
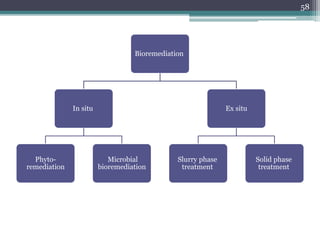
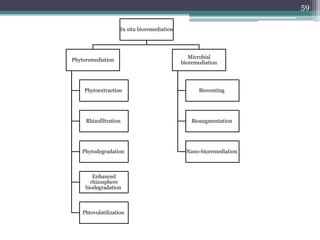
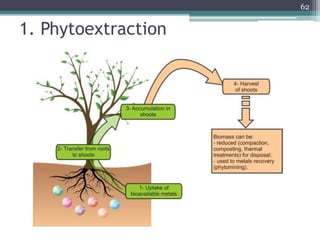
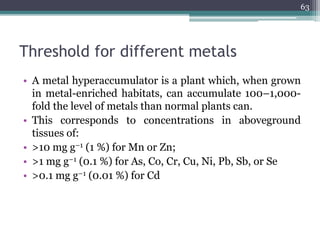
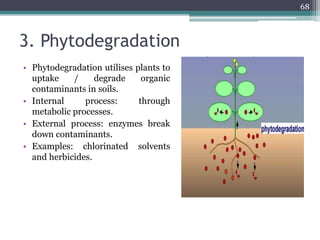
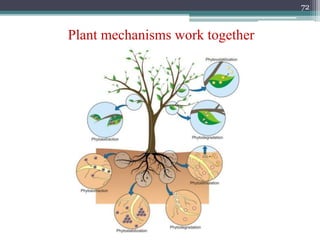
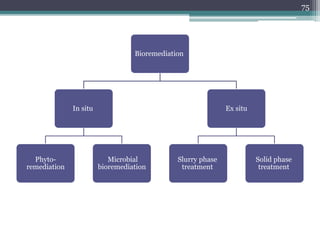
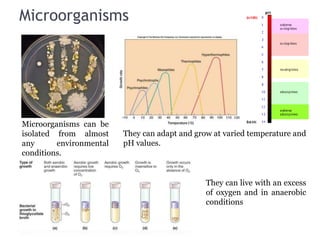
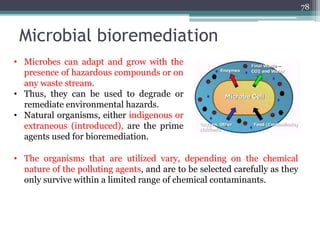
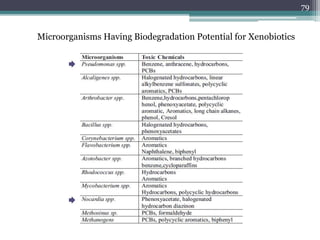
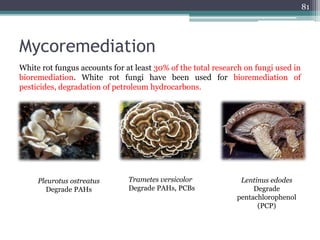
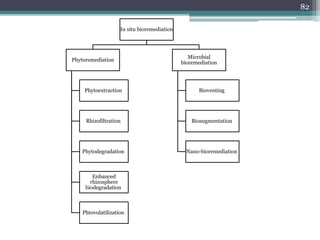
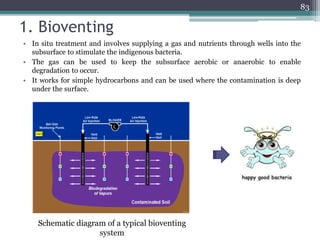
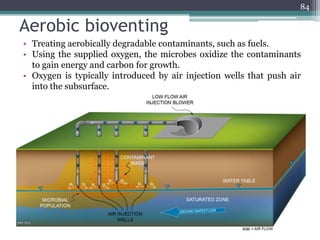
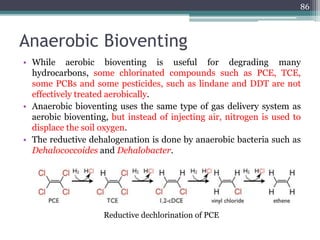
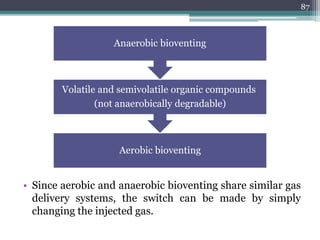
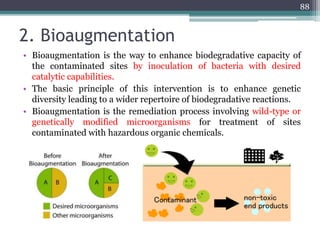
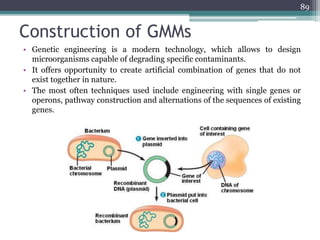
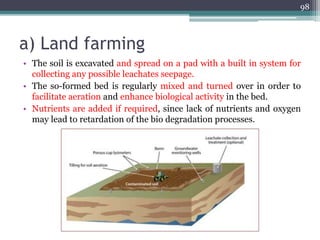
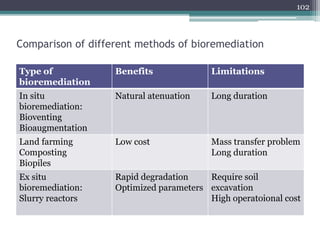
The document discusses seven primary sources of soil pollution including industrial waste, agricultural practices, biological agents, mining, radioactive pollutants, acid rain, and urban waste, detailing their harmful effects on soil quality and health. It highlights the consequences of soil contamination on fertility, human health, and ecosystems, emphasizing issues like heavy metal toxicity and the persistence of pesticides. Remediation technologies such as oxidation, ion exchange, and soil washing are also outlined as methods for addressing soil pollution.