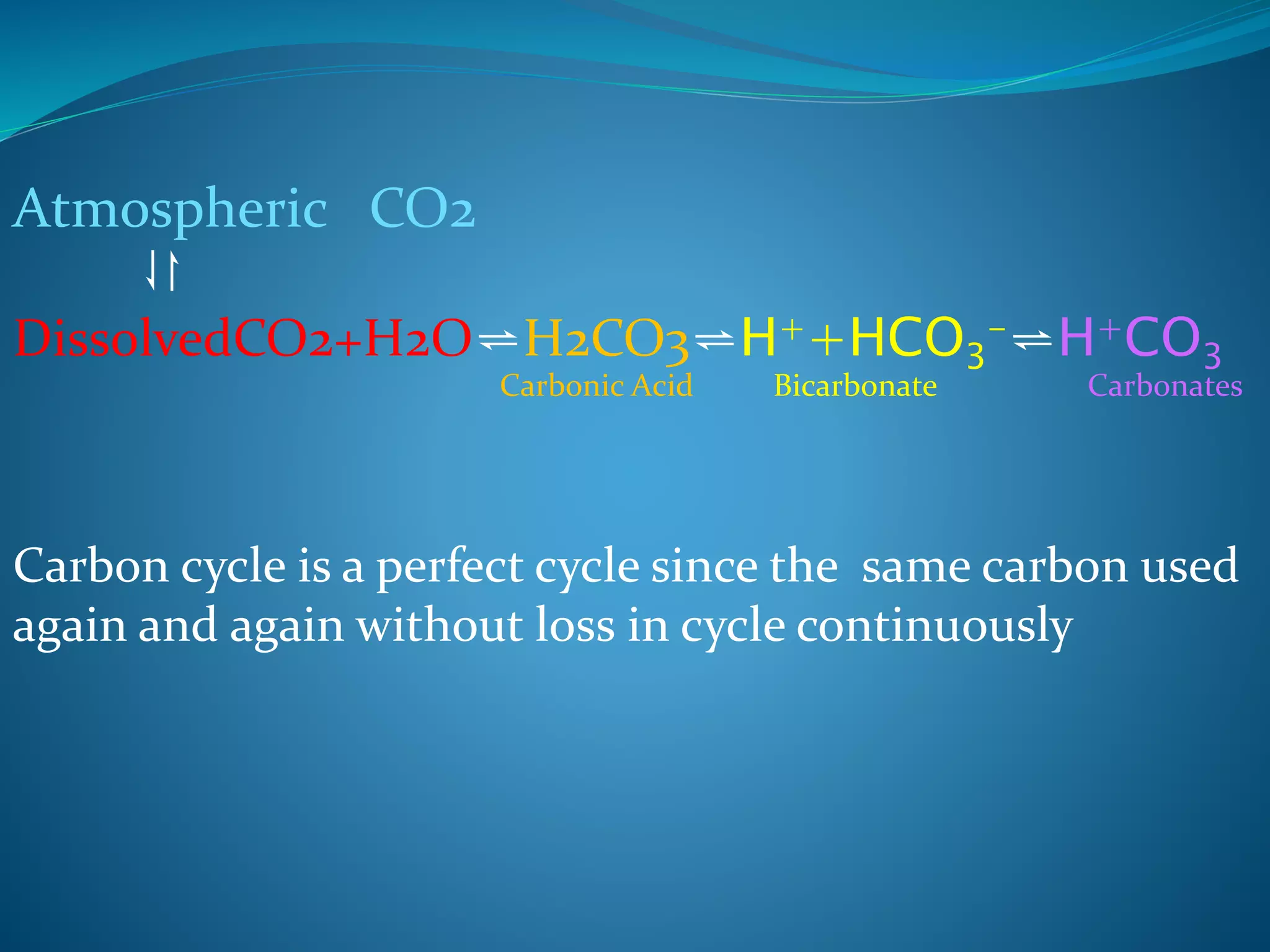
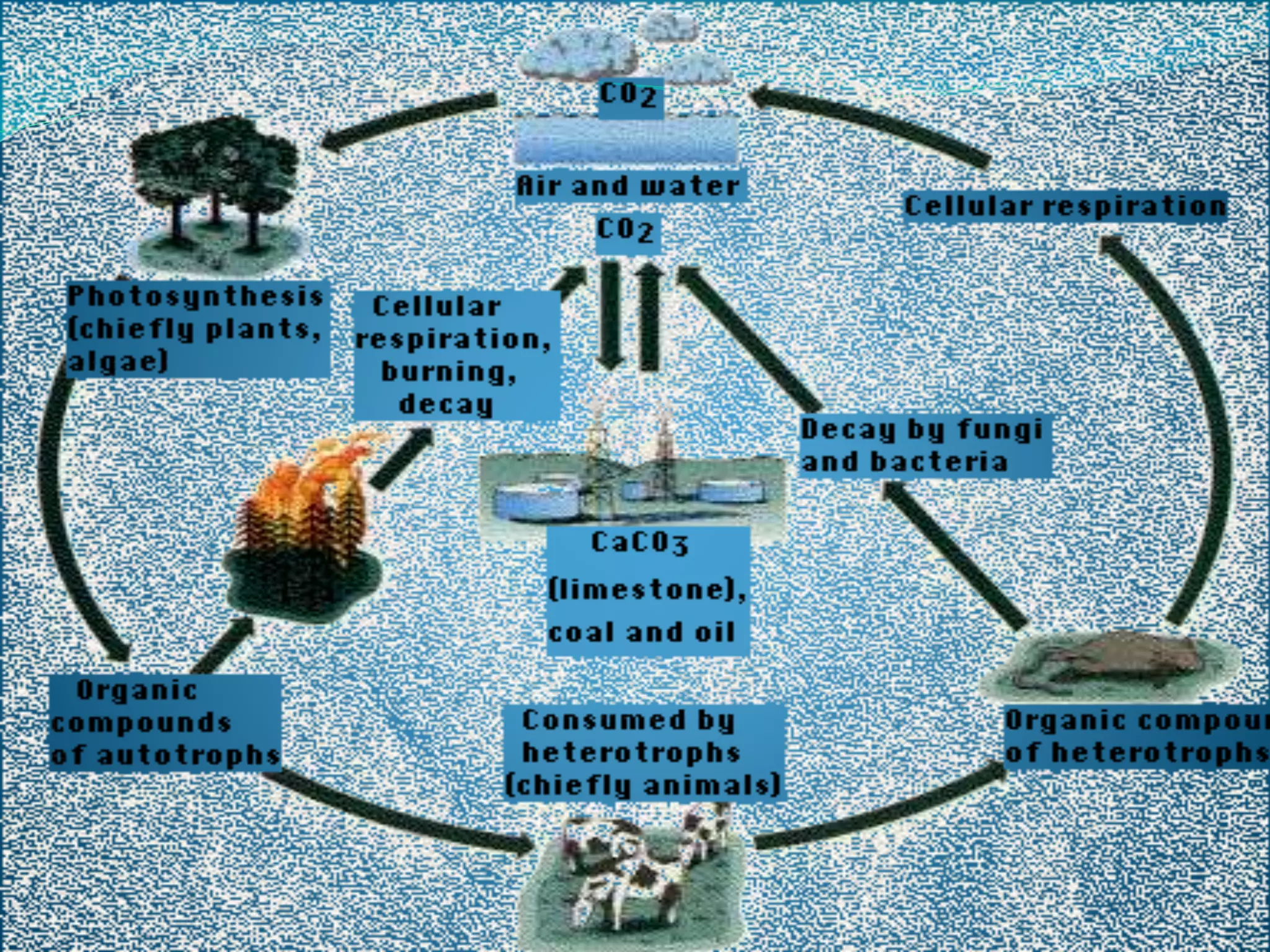
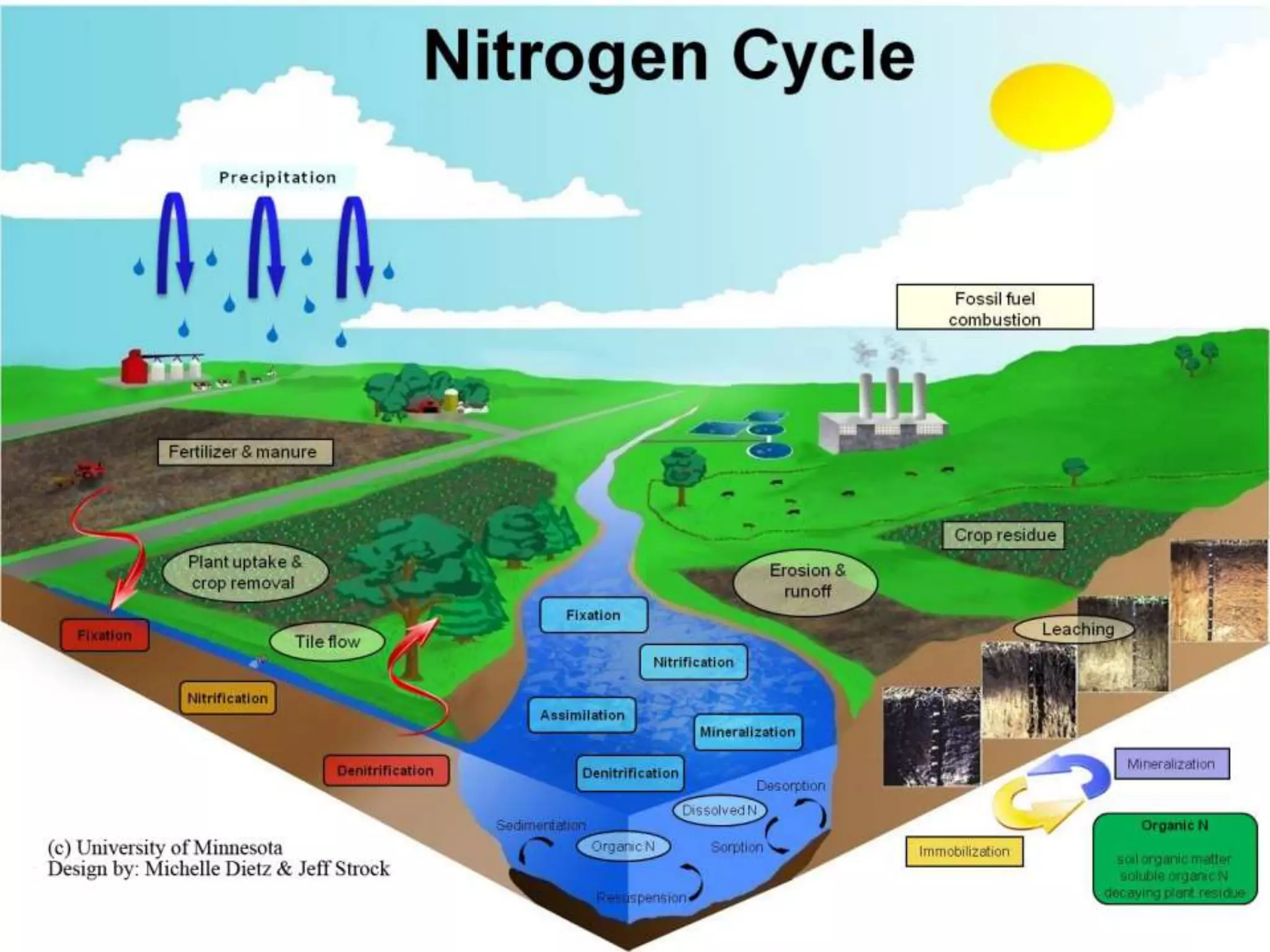
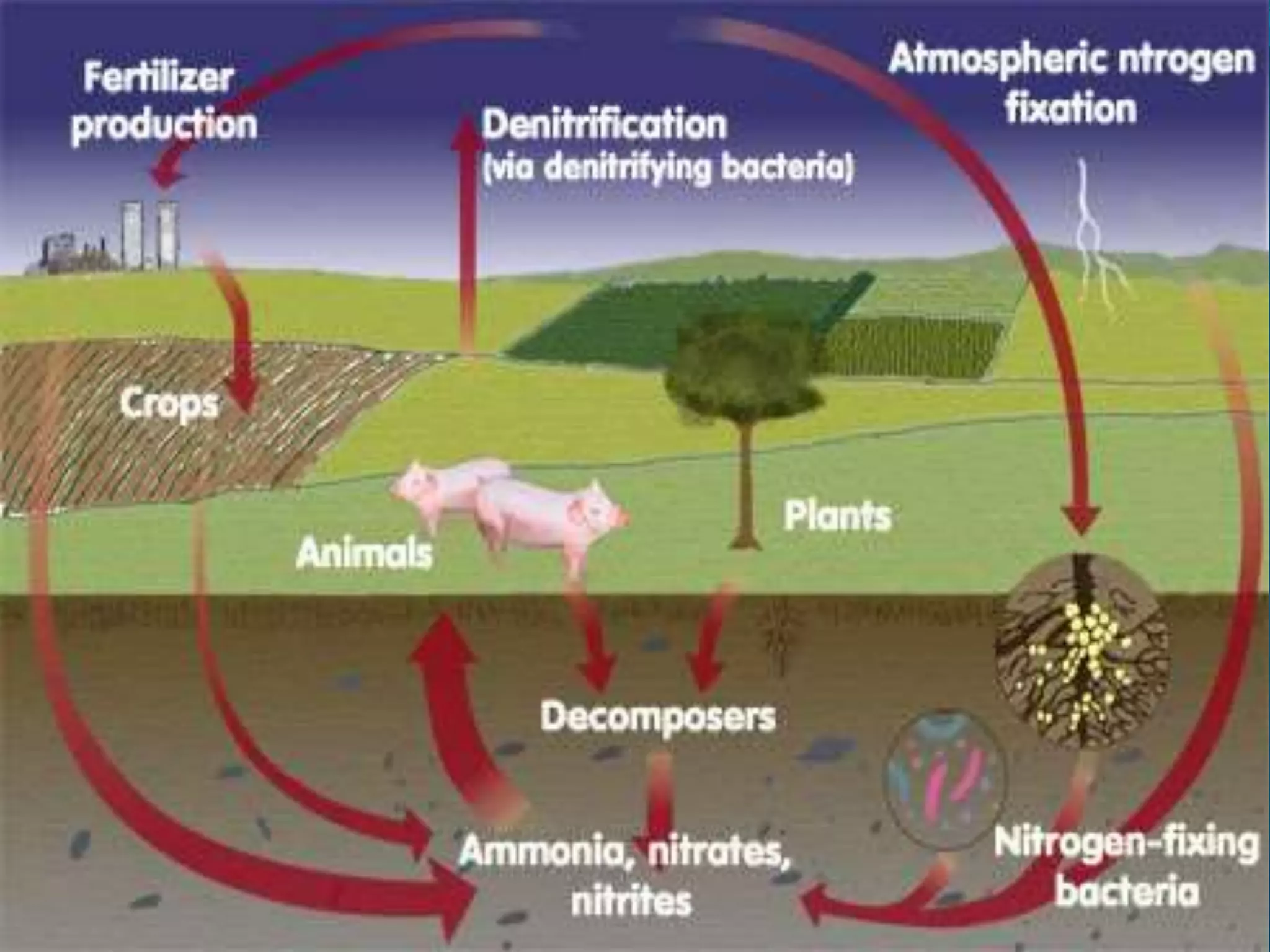
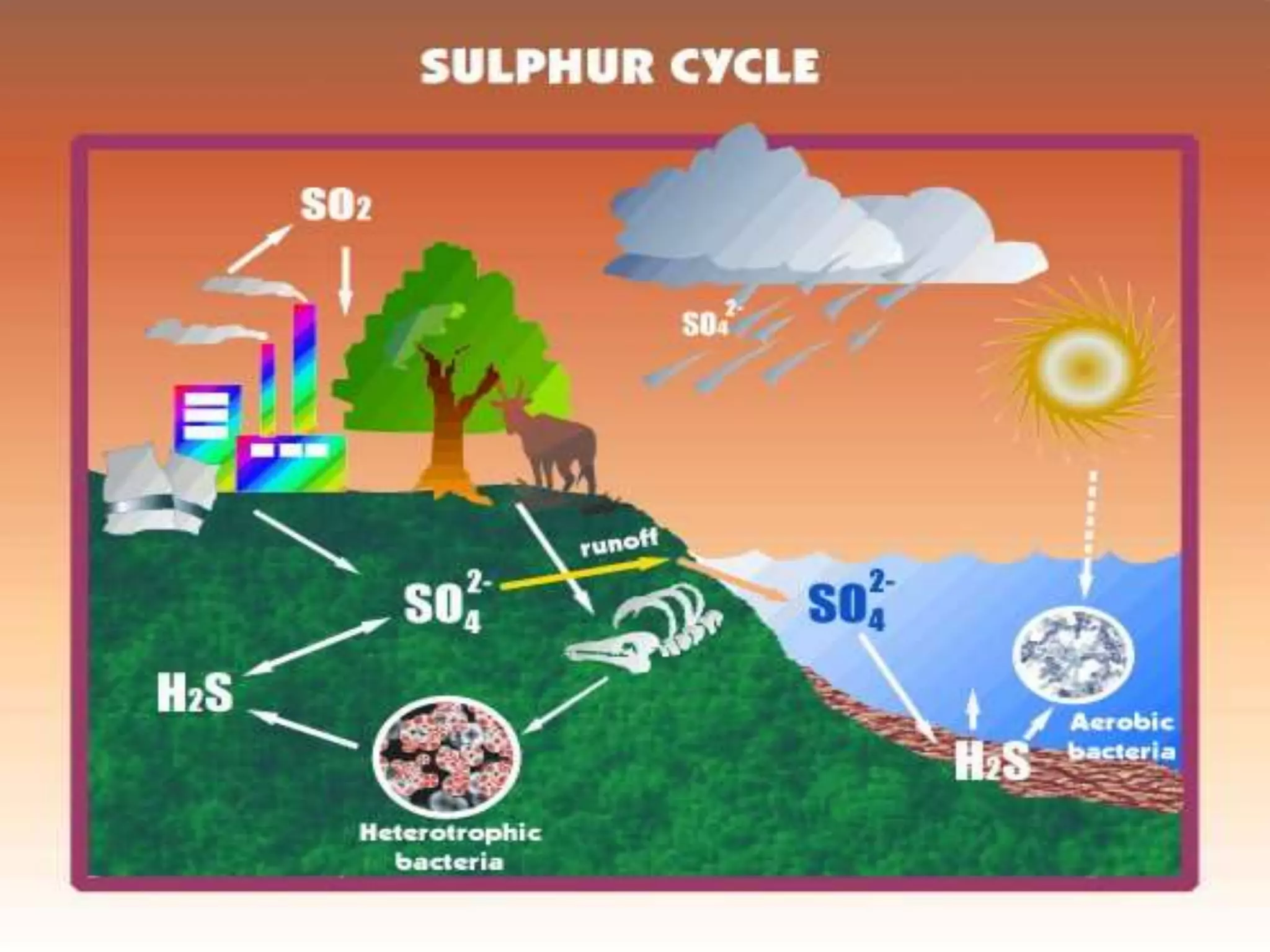
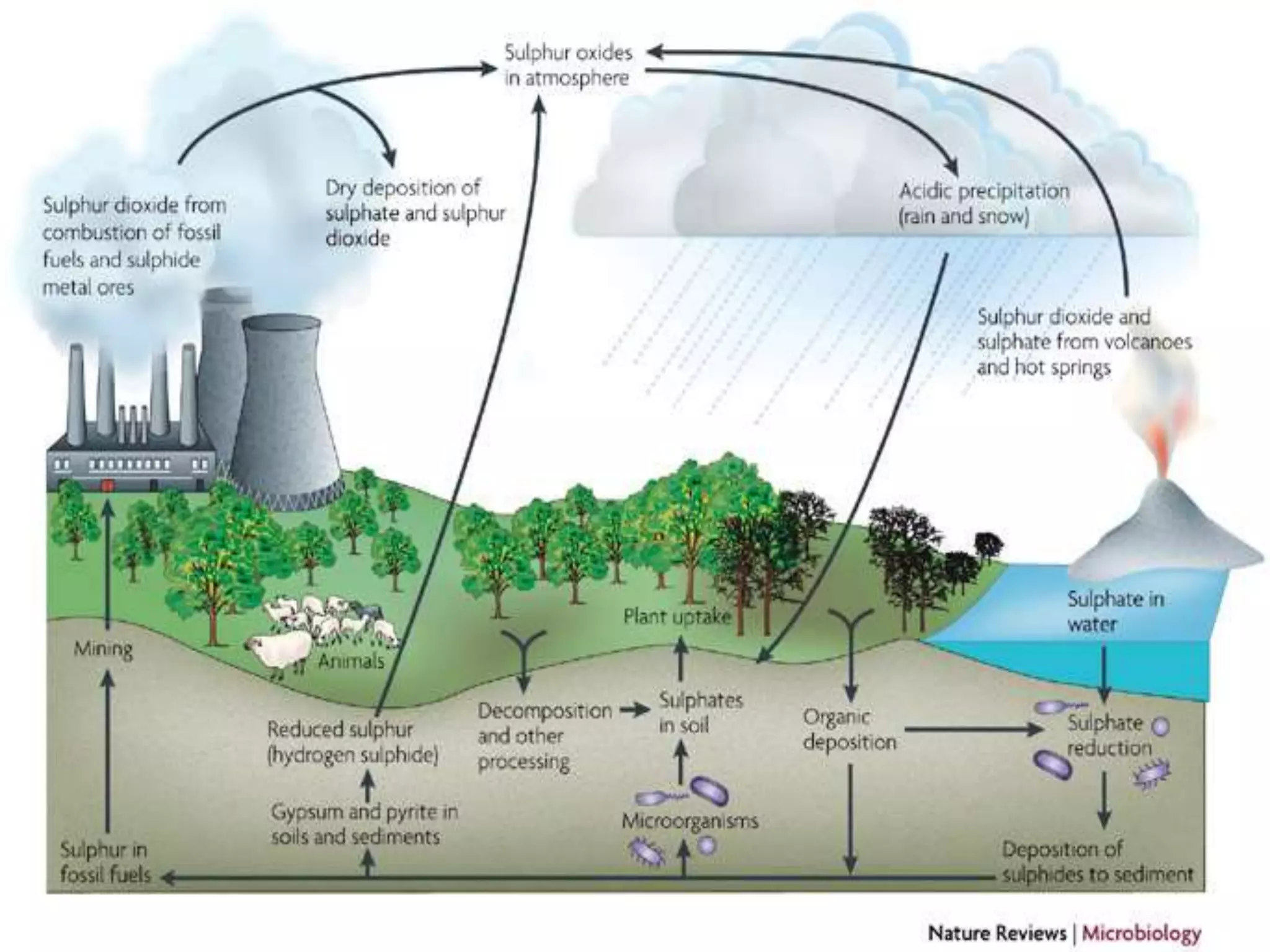
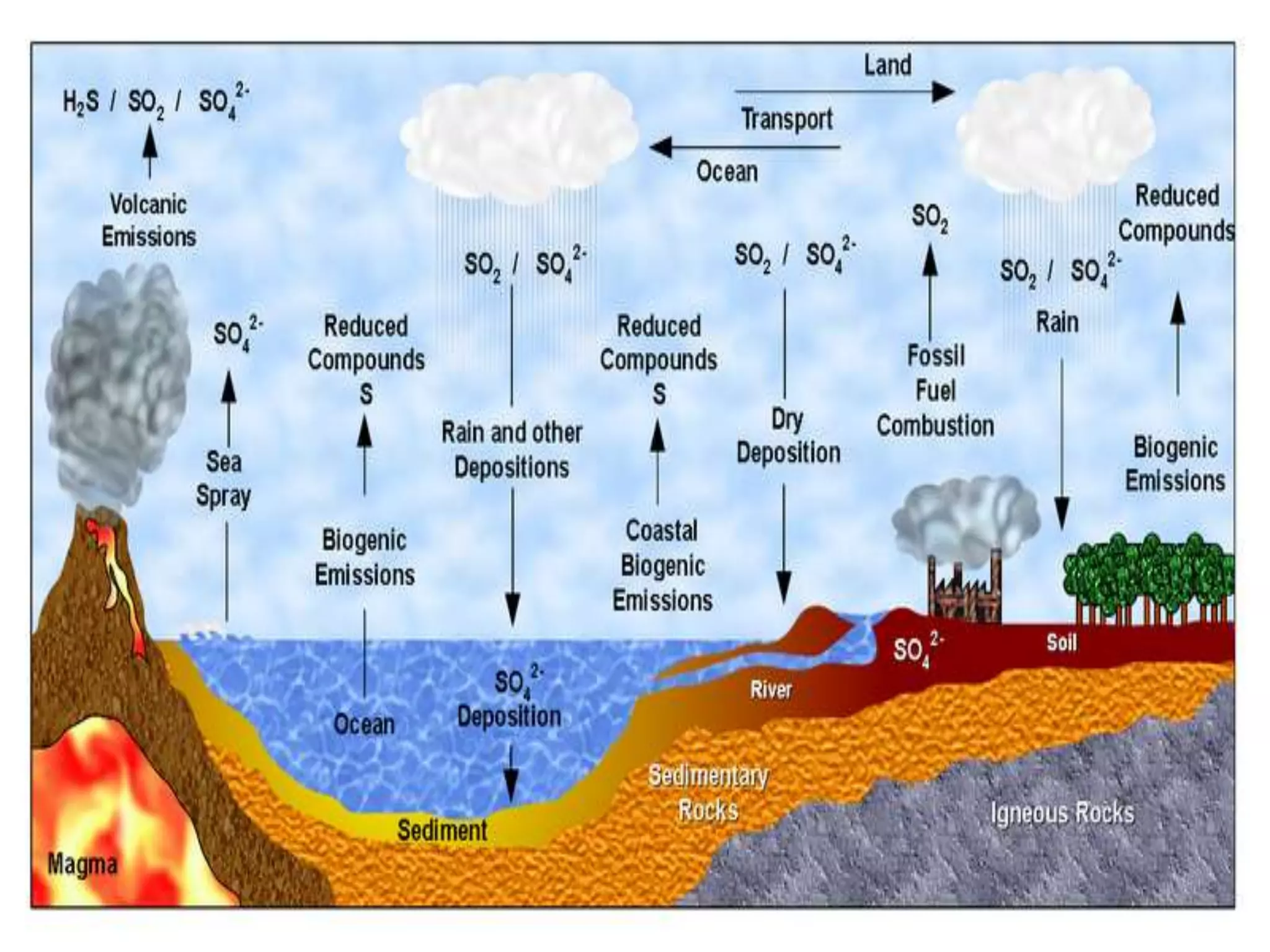
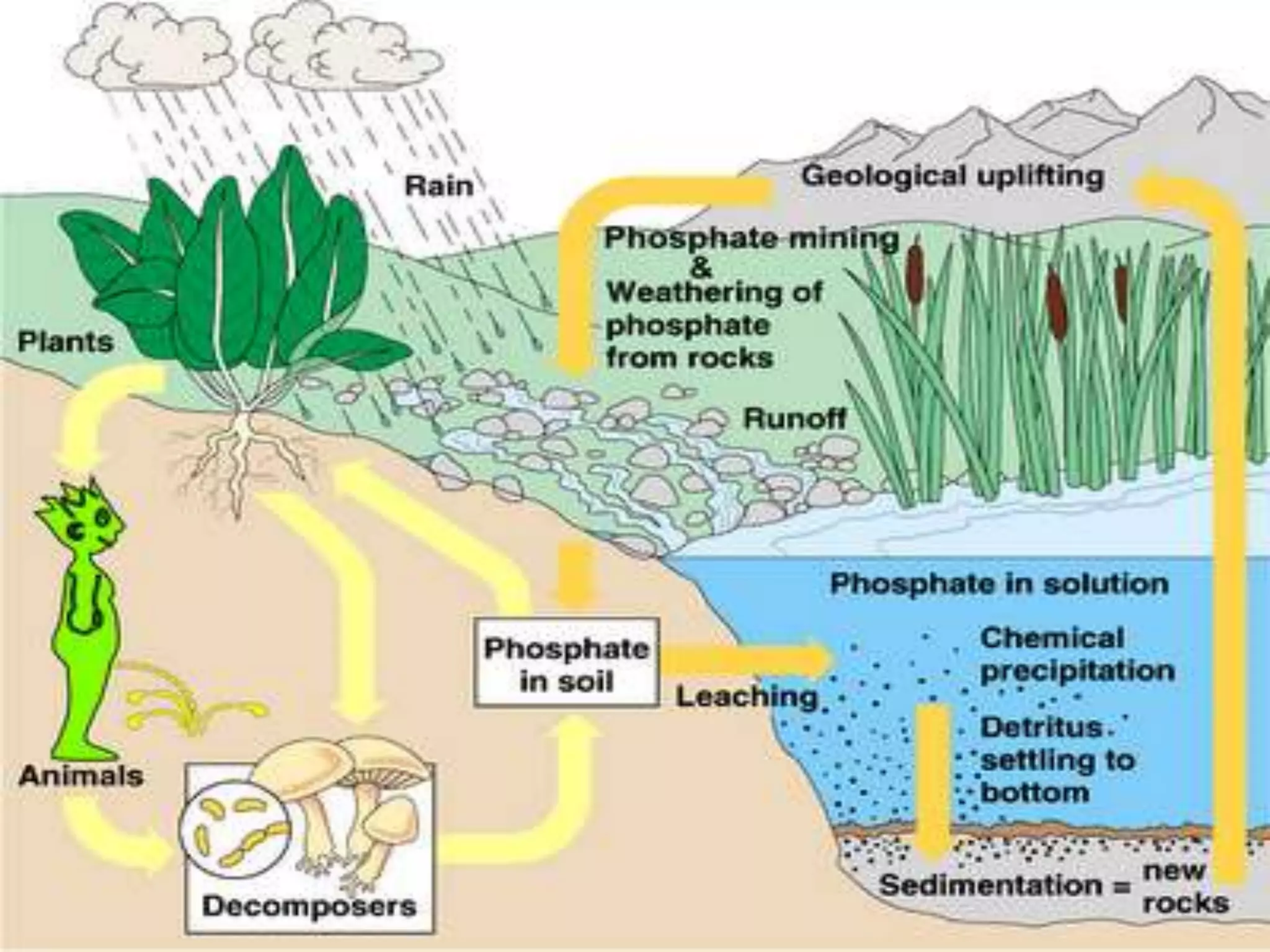
The document discusses biogeochemical cycles, which describe how chemical elements move through biotic and abiotic components of ecosystems. It describes several important cycles, including the carbon, nitrogen, sulfur, and phosphorus cycles. The carbon and nitrogen cycles involve gaseous forms and are considered closed or perfect cycles. The sulfur and phosphorus cycles are sedimentary and imperfect, as elements are eventually deposited as sediments. Biogeochemical cycles sustain life by recycling essential elements and maintaining chemical balances in ecosystems.





![Most biogeochemical cycles are described as elemental
cycles involving nutrient elements such as carbon [c],
Nitrogen [N], Oxygen [O], Phosphorus [P] and Sulphur [S].
Many are exogenic cycles in which the element
concerned spend parts of the cycle in the atmosphere-
O2,N2,C and CO2.
P do not have the gaseous component and it is in
Endogenic cycles.
ENDOGENIC AND EXOGENIC CYCLES](https://image.slidesharecdn.com/anilaslideshare-200812072652/75/Biogeochemical-Cycle-6-2048.jpg)



![Most important gaseous cycles are,
Water cycle [Hydrological Cycle]
Carbon Cycle
Oxygen Cycle
Nitrogen Cycle](https://image.slidesharecdn.com/anilaslideshare-200812072652/75/Biogeochemical-Cycle-10-2048.jpg)