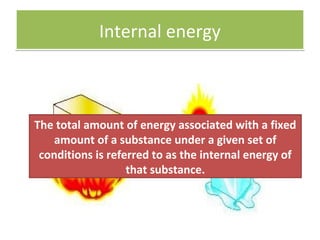
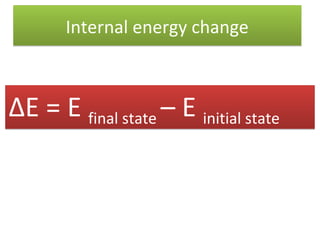
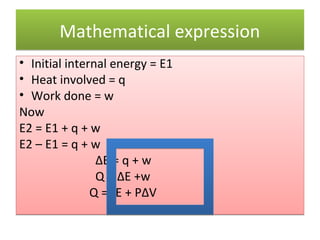
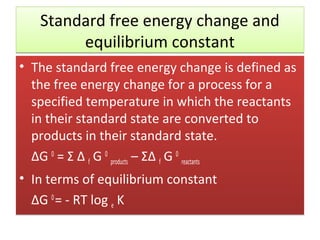
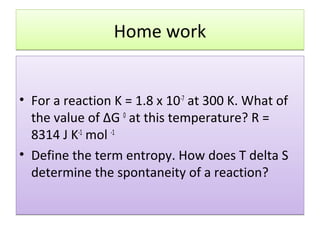
Here are the answers to your homework questions:
1) For a reaction K = 1.8 x 10-7 at 300 K. What of the value of ΔG 0 at this temperature?
R = 8314 J K-1 mol -1
ΔG0 = -RT ln K
= - (8314 J K-1 mol-1) (300 K) ln (1.8 x 10-7)
= +21.3 kJ mol-1
2) Entropy is a measure of the randomness or disorder of a system. A positive change in entropy (ΔS) favors spontaneity for a process at constant temperature and pressure. The factor TΔS in the expression for Gibbs