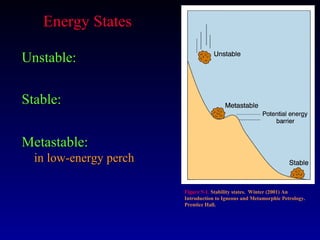
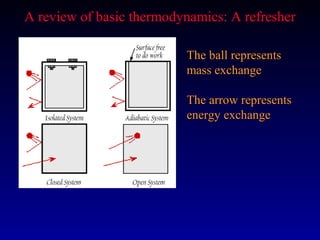
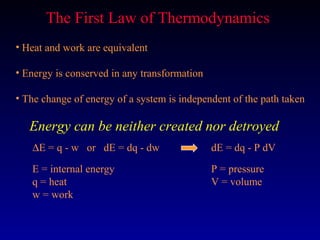
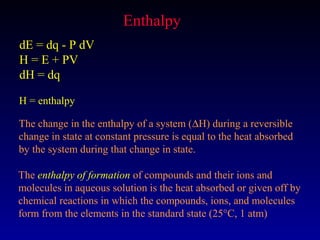
Thermodynamics studies energy changes that occur in systems and their surroundings. A system is the portion of the universe being studied, while the surroundings are everything outside the system. Natural systems tend toward states of minimum energy. The first law of thermodynamics states that energy is conserved during any process. The second law states that the entropy of the universe increases over time as energy moves from being concentrated to becoming dispersed.