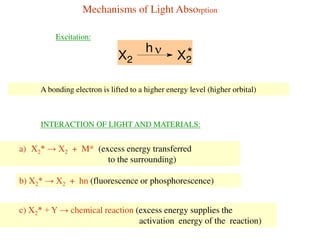
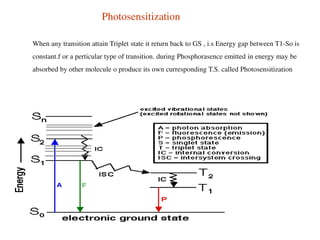
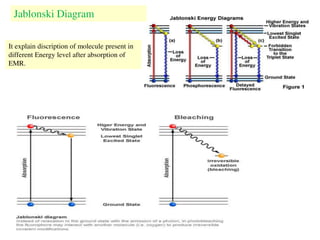
This document summarizes a seminar on photochemistry presented by Mr. Dinkar B. Kamkhede. The seminar covered topics including the definition of photochemistry, laws of photochemistry, mechanisms of light absorption, electronic transitions, photosensitization, and the Jablonski diagram. It discussed how photochemical reactions are initiated by the absorption of light energy and explained concepts such as quantum yield. The seminar provided an overview of the key concepts and processes in photochemistry.



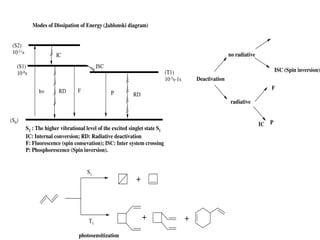
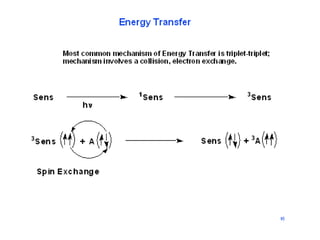
![Energy transfer through photosensitization
D 1D
h
1D 3D
ISC
A + 3D D + 3A
3A Products
D = Donor
A = Acceptor
1 = Singlet
3 = Triplet
S0
S1
74 Kcal
.mole-1 69 Kcal/mole
T1
ISC
120 Kcal/mole
S0
T1
S1
60 Kcal/mole
Energy transfer
Benzophenone Butadiene
Ph2CO
h
1[Ph2CO]
ISC 3[Ph2CO]
+ Ph2CO
3
Dimeric products](https://image.slidesharecdn.com/dinkarpotochemistryppt-150310035758-conversion-gate01/85/Dinkars-presentation-on-potochemistry-11-320.jpg)