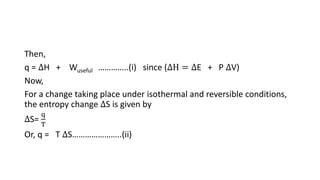
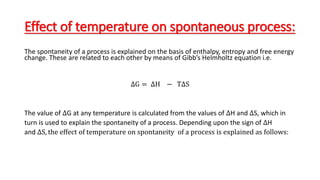
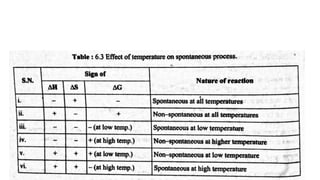
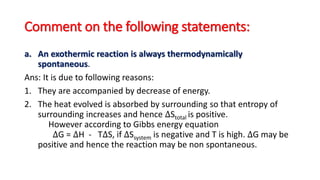
This document discusses spontaneous processes and the driving forces behind them in thermodynamics. It explains that spontaneous processes are driven by a decrease in enthalpy or an increase in entropy. While enthalpy change alone cannot predict spontaneity, the introduction of entropy and Gibbs free energy allows better determination of spontaneous processes. The document also discusses how temperature, entropy change, and the relationship between Gibbs free energy and equilibrium constant can be used to analyze spontaneity.















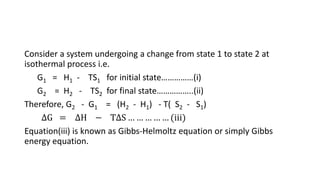

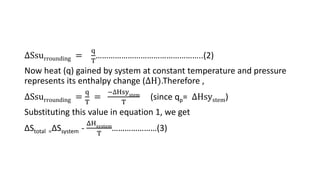
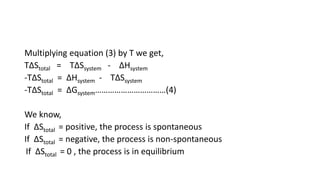

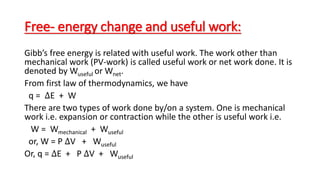



![Standard free energy change:
The standard free energy change is defined as the free energy change
for a process at 298 K and 1 atmospheric pressure in which the
reactants in their standard states are converted to the products in their
standard states. Thus,
Δ G° = Σ Δ G °(products) - Σ Δ G °(reactants)
=[sum of the standard free energy in the formation of products ] –
[ sum of standard energy in the formation of reactants]](https://image.slidesharecdn.com/thermodynamicsii-180130054401/85/Thermodynamics-ii-37-320.jpg)
