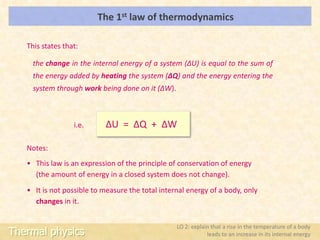
This document discusses the internal energy unit of an A-Level Physics course on thermal physics. It includes learning objectives about defining internal energy, how temperature increases relate to internal energy increases, and using the first law of thermodynamics. Specific topics covered include the internal energy of an ideal gas, changes in internal energy during phase changes, and demonstrations of work being done to change internal energies.