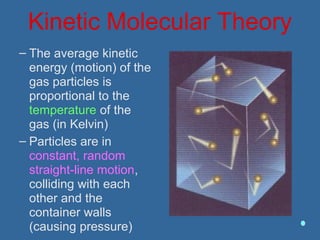
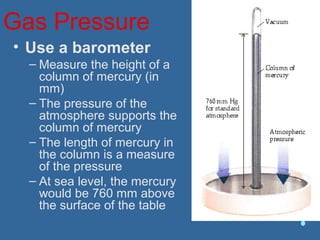
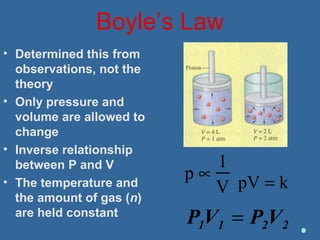
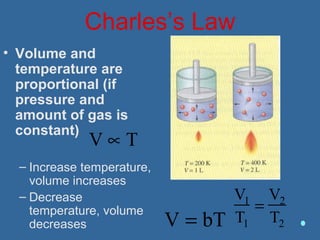
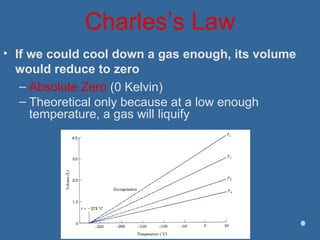
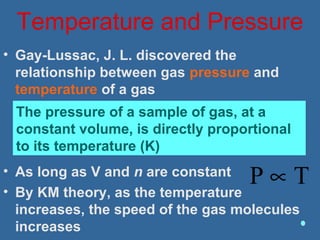
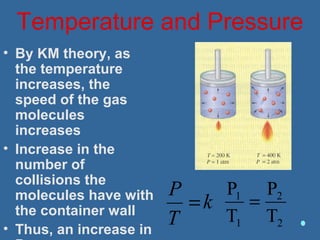
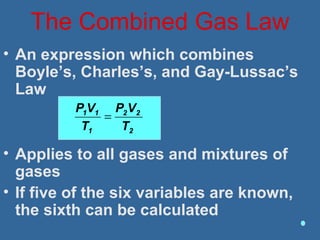
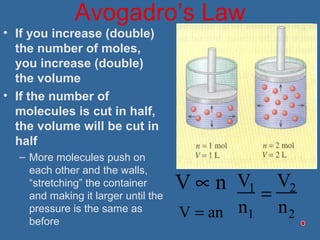
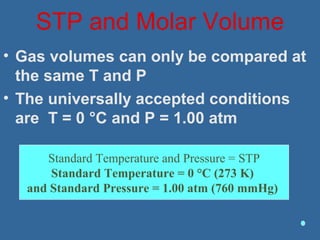
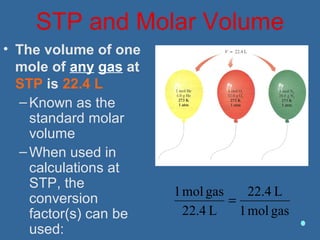
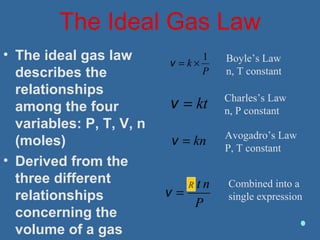
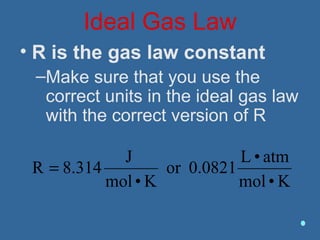
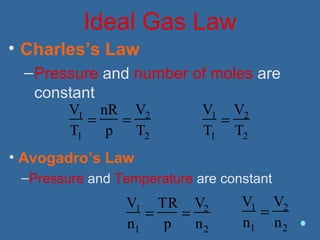
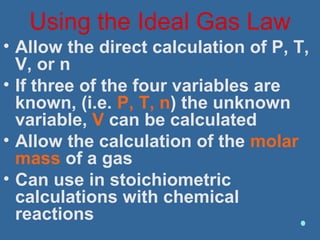
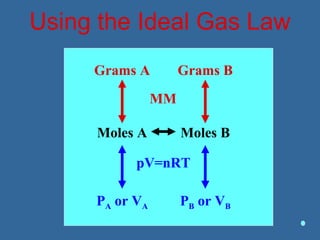
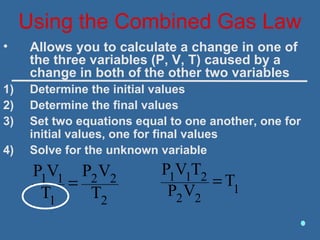
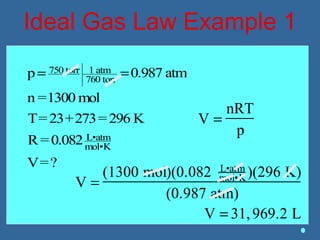
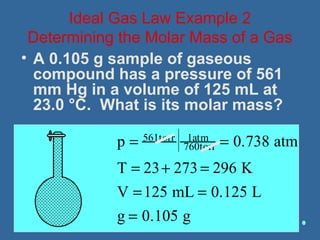
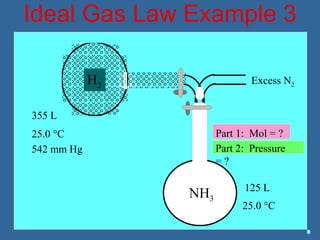
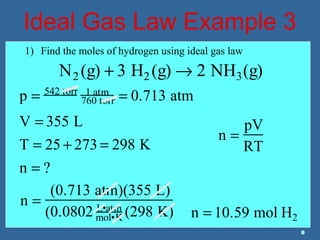
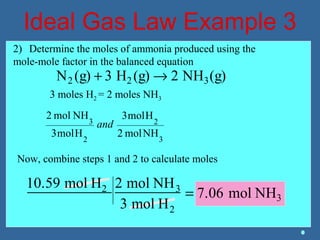
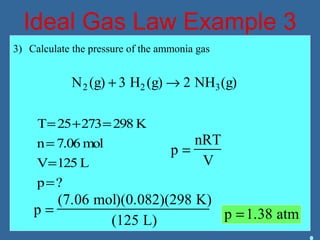
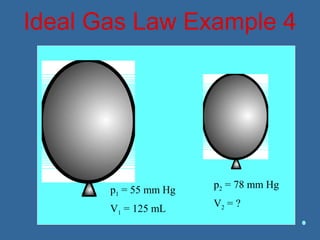
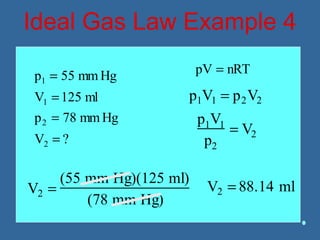
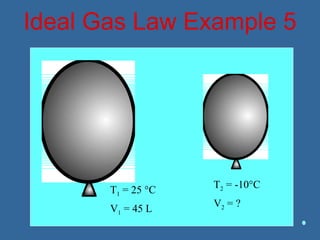
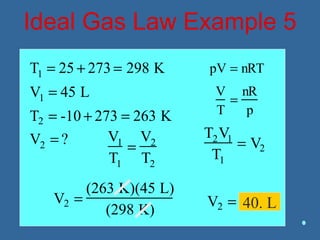
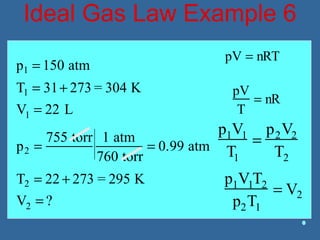
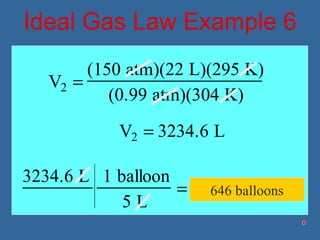
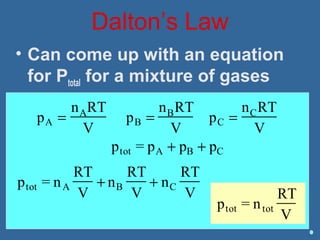
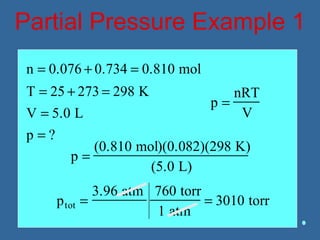
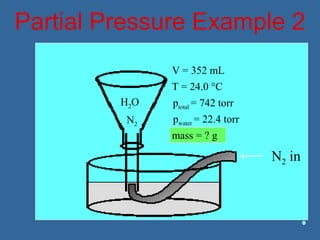
Gases are composed of tiny particles that are in constant, random motion. Three properties of gases are pressure, volume, and temperature. The kinetic molecular theory and gas laws describe the relationships between these properties. The ideal gas law combines earlier gas laws relating pressure, volume, amount of gas, and temperature into a single equation.