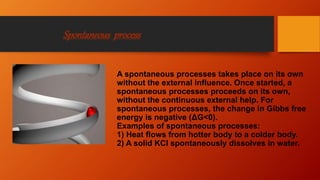
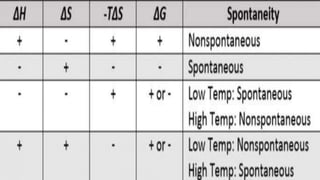
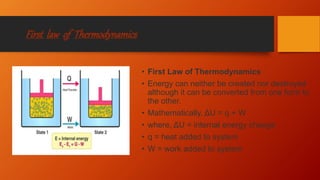
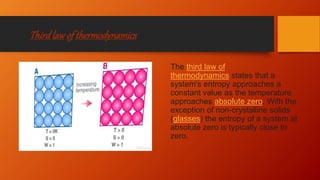
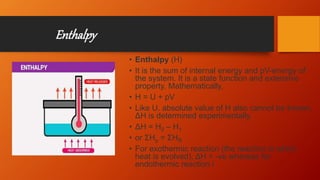
The document provides an overview of key concepts in thermodynamics. It defines thermodynamics as the branch of science dealing with quantitative relationships between heat and other forms of energy. It then discusses important terms, types of systems, thermodynamic properties and processes, laws of thermodynamics, and other concepts such as internal energy, enthalpy, entropy, spontaneous processes, and Gibbs free energy.




















![Expression for p-vWork
• Expression for Pressure – Volume Work
• (i) Work of Irreversible expansion against constant pressure B under isothermal conditions
• WpV = – pext ΔV
• (iv) Work of irreversible expansion under adiabatic conditions
• (v) When an ideal gas expands in vacuum then
• pext = 0
• Work done is maximum in reversible conditions
• Units CGS system – erg
• SI system – joule
• Work and heat both appear only at the boundary of the system during a change in state.]](https://image.slidesharecdn.com/jatinbhatiaartintegrationproject22-210120050151/85/Jatin-bhatia-art-integration-project-22-25-320.jpg)