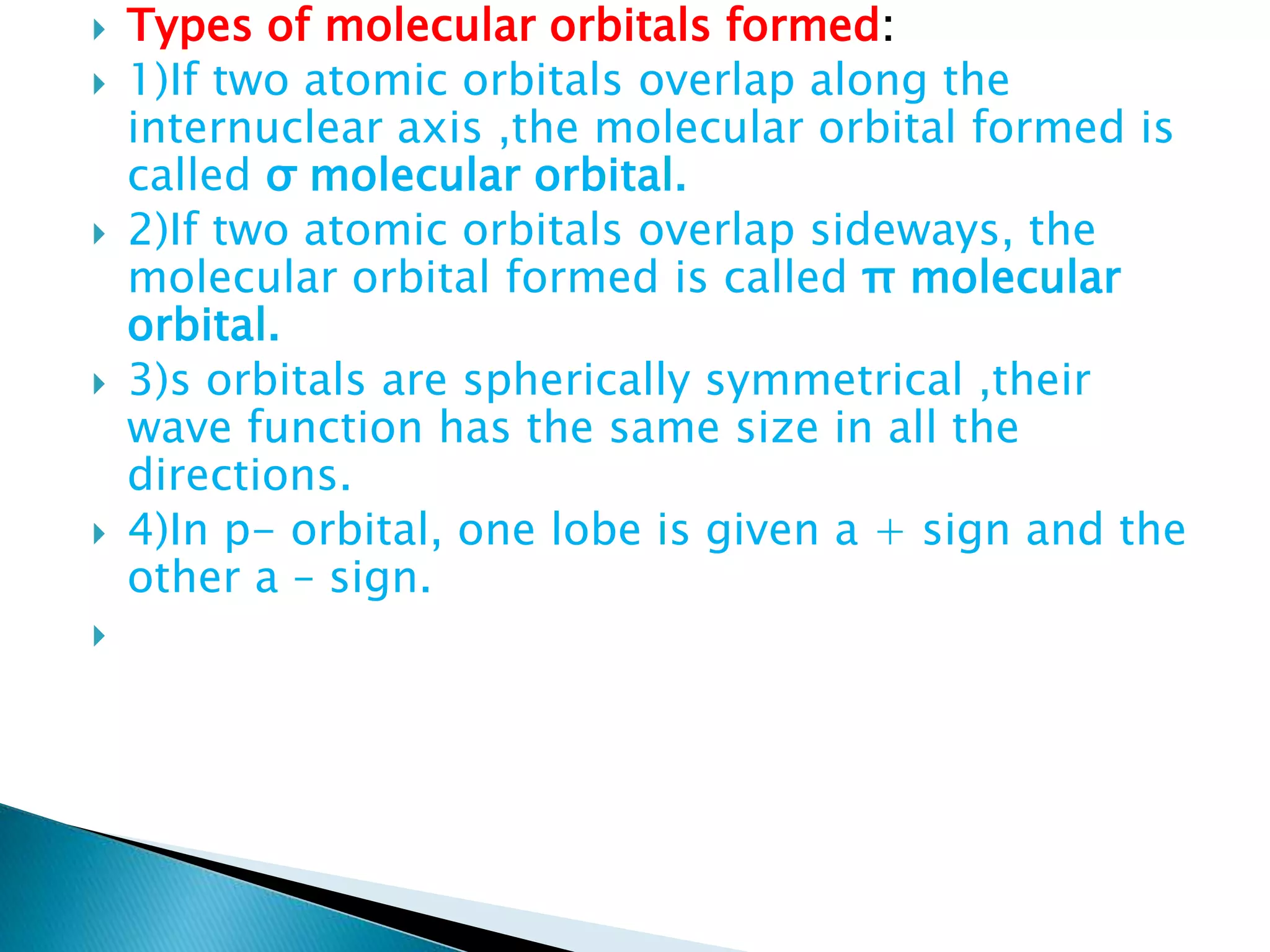
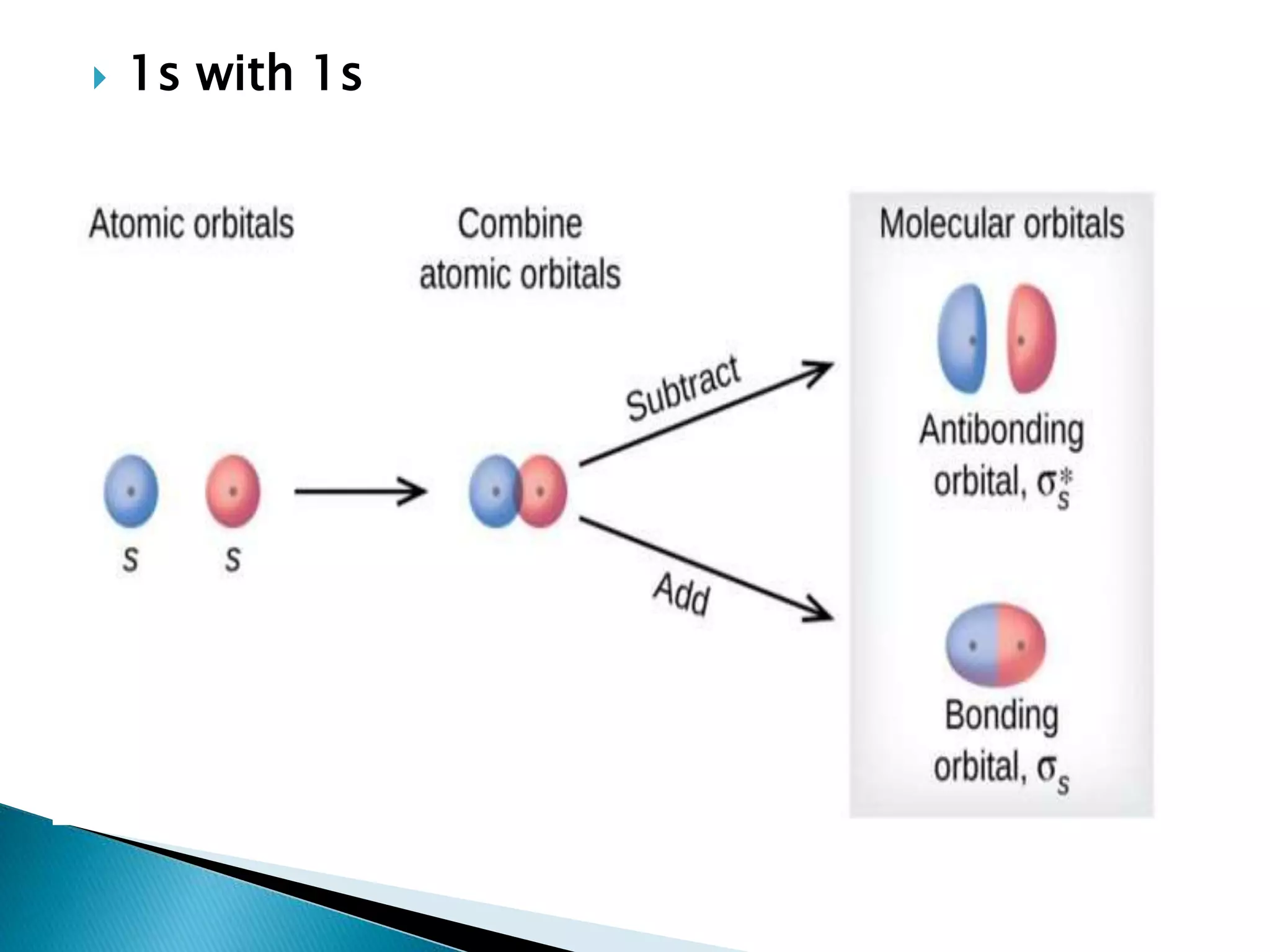
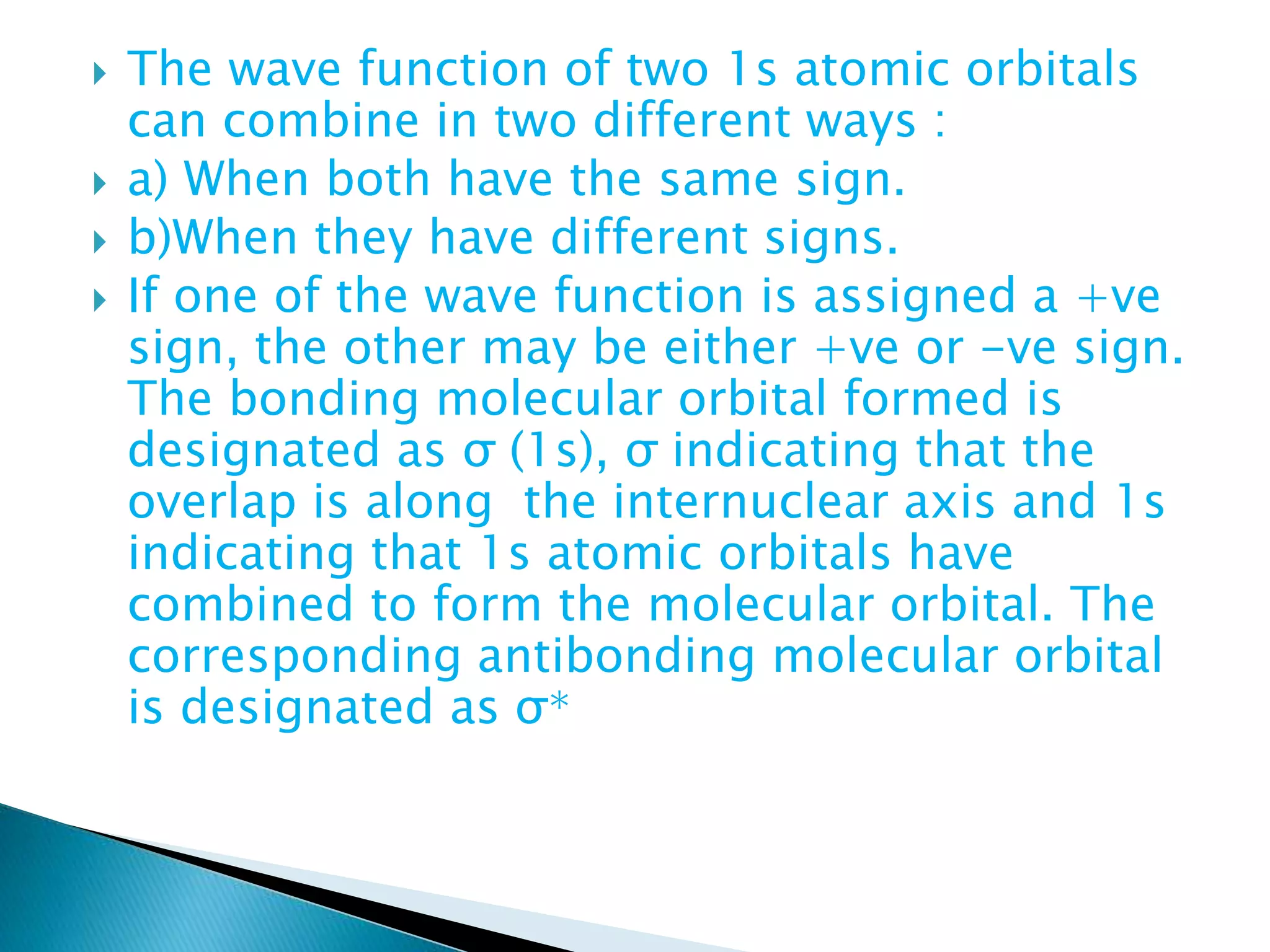
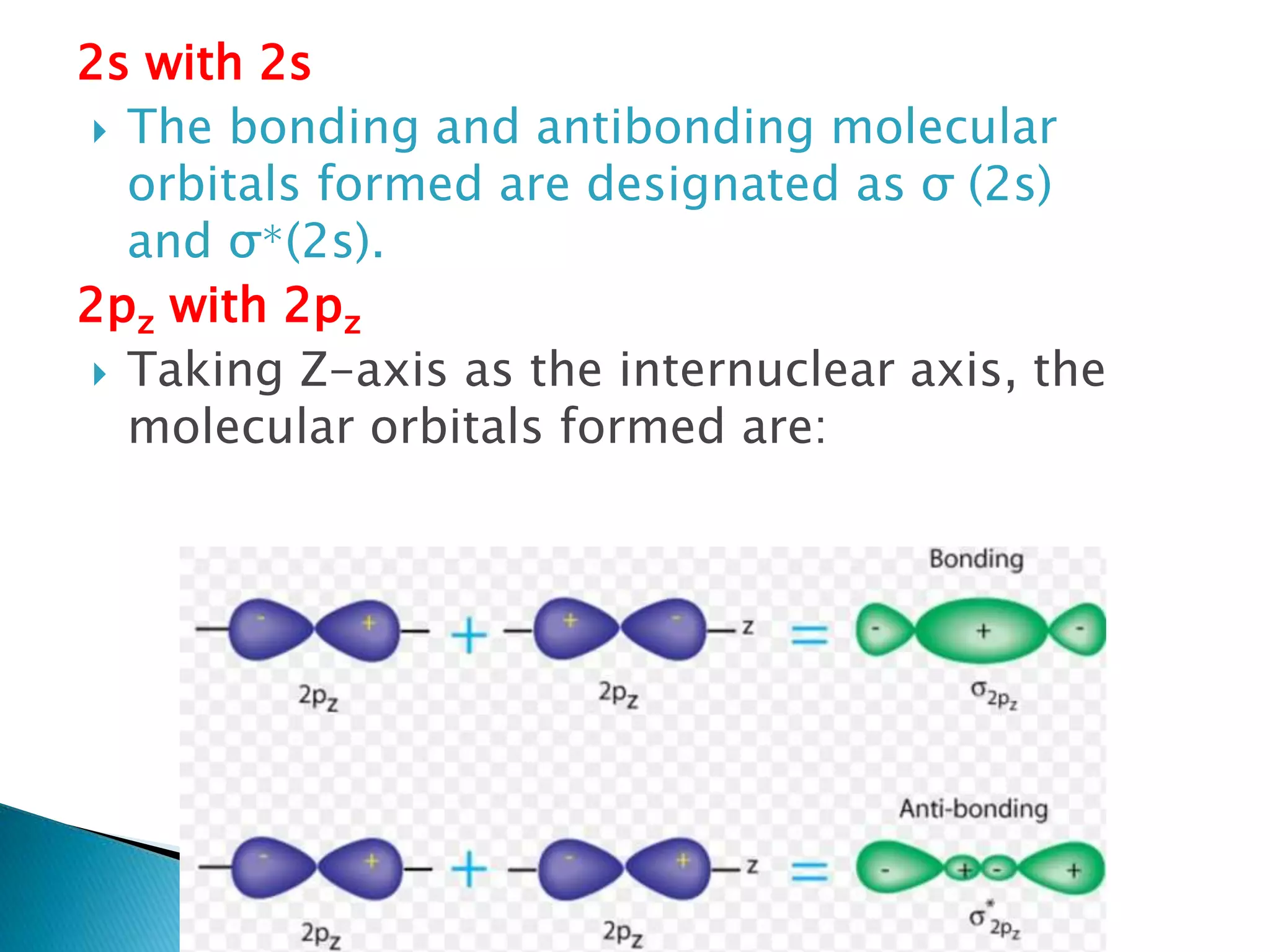
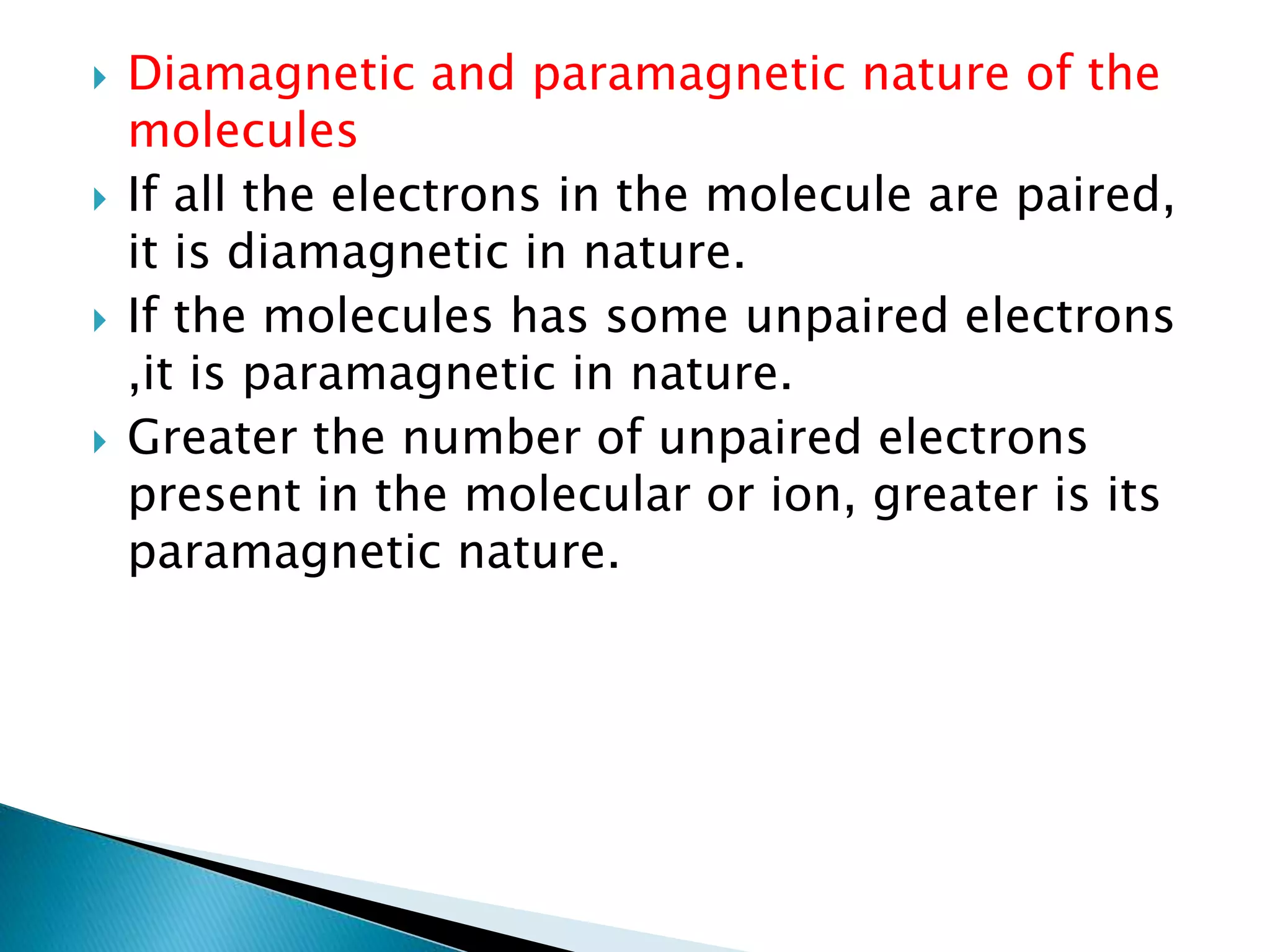
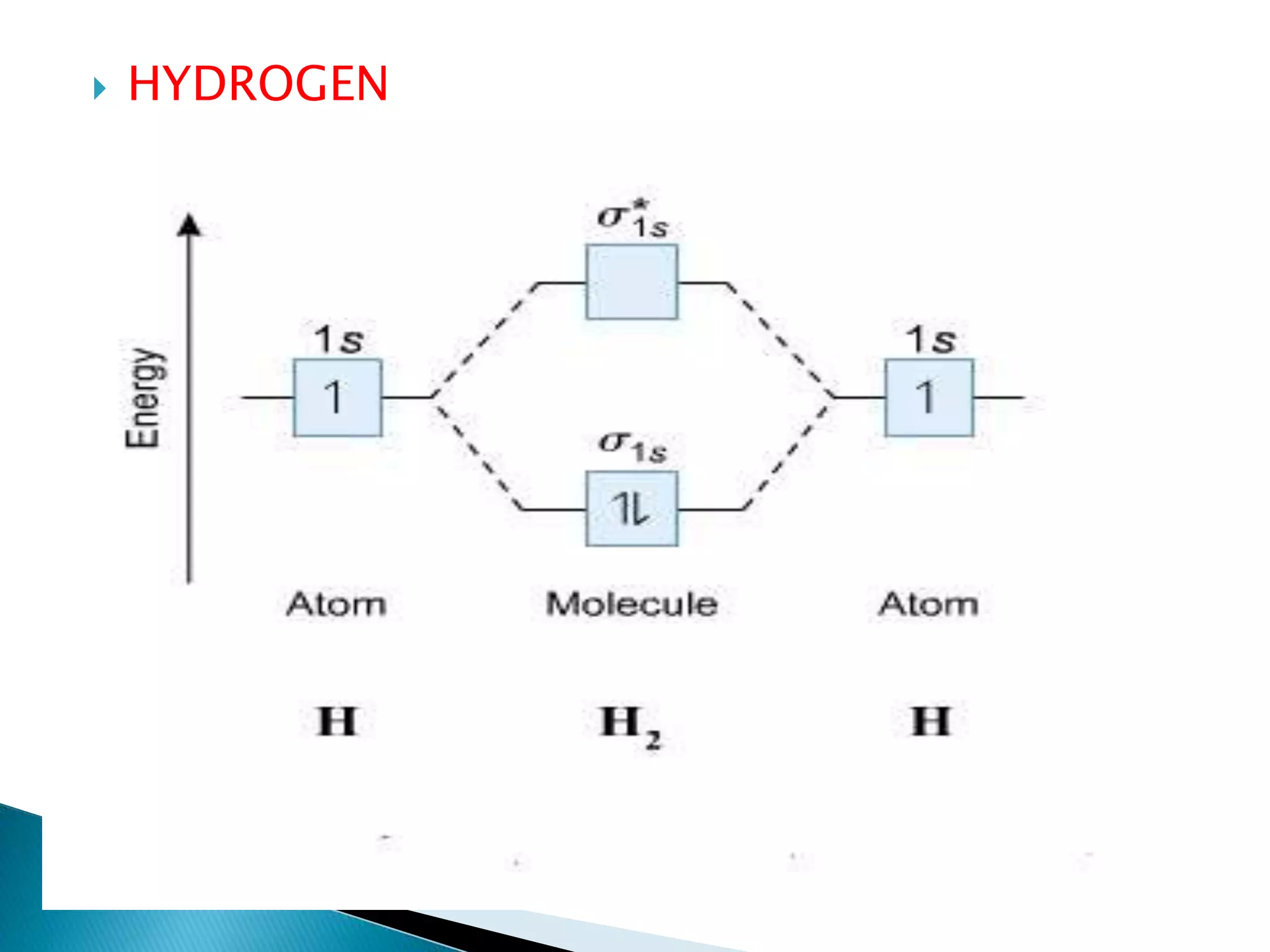
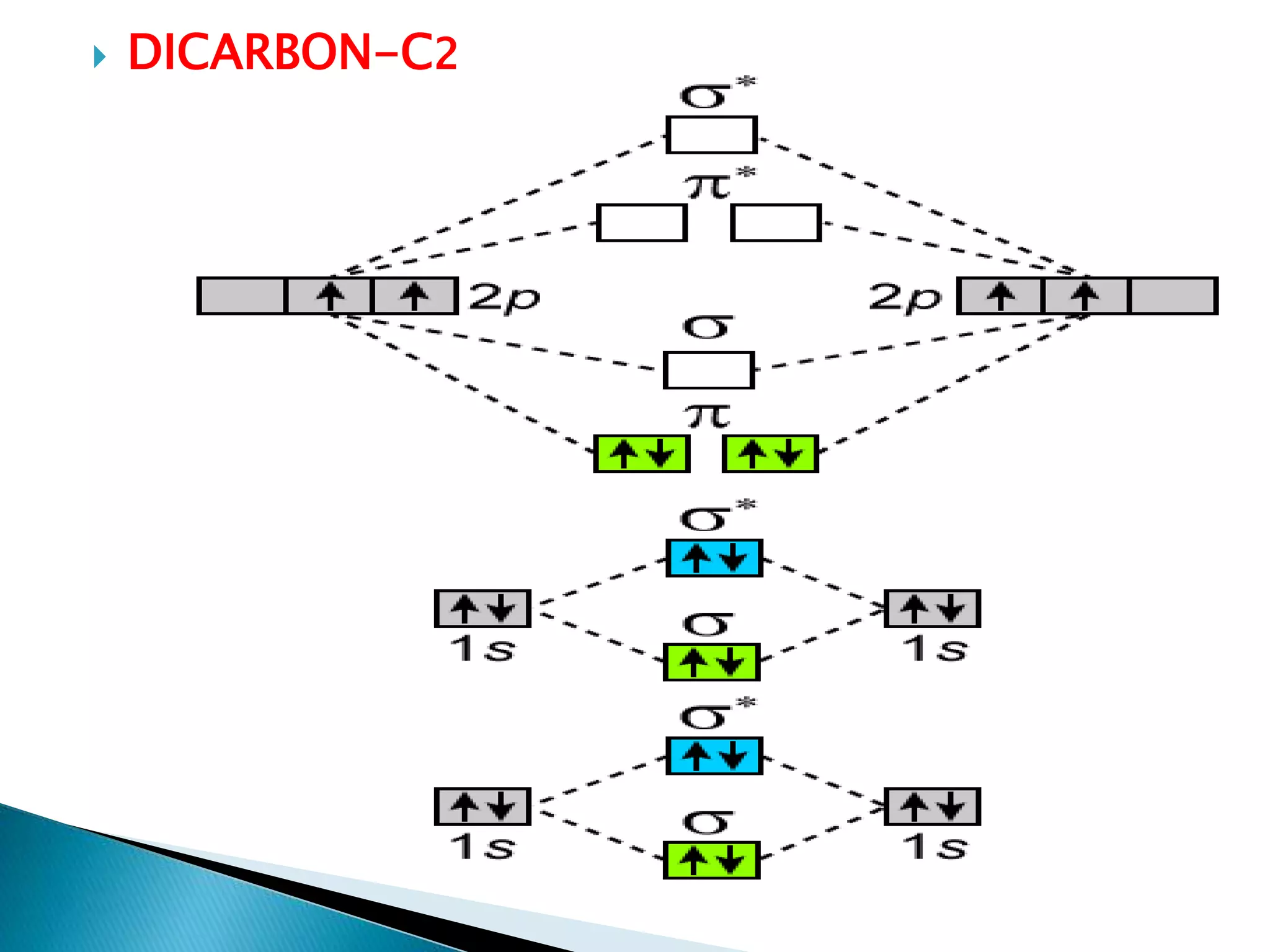
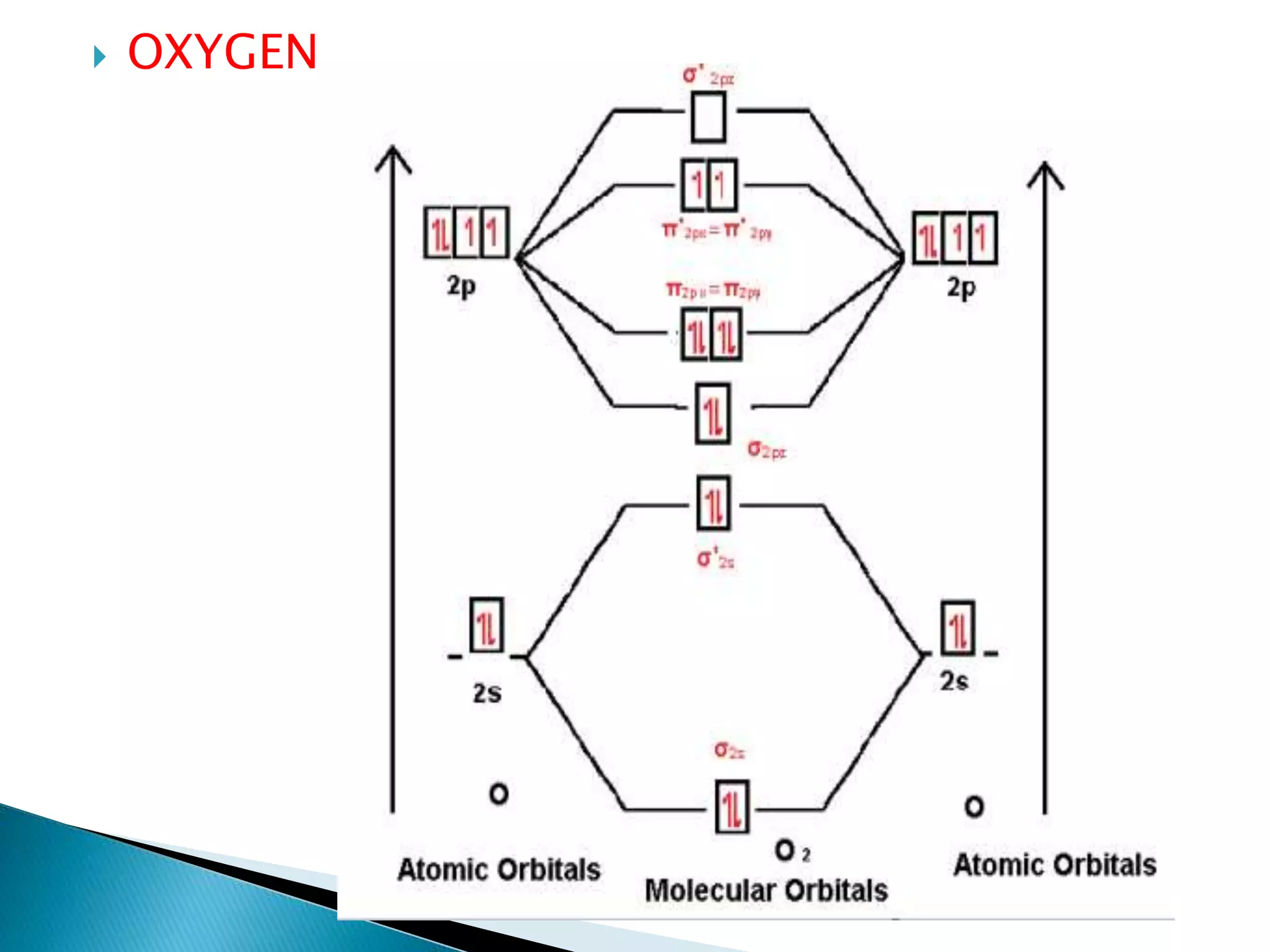
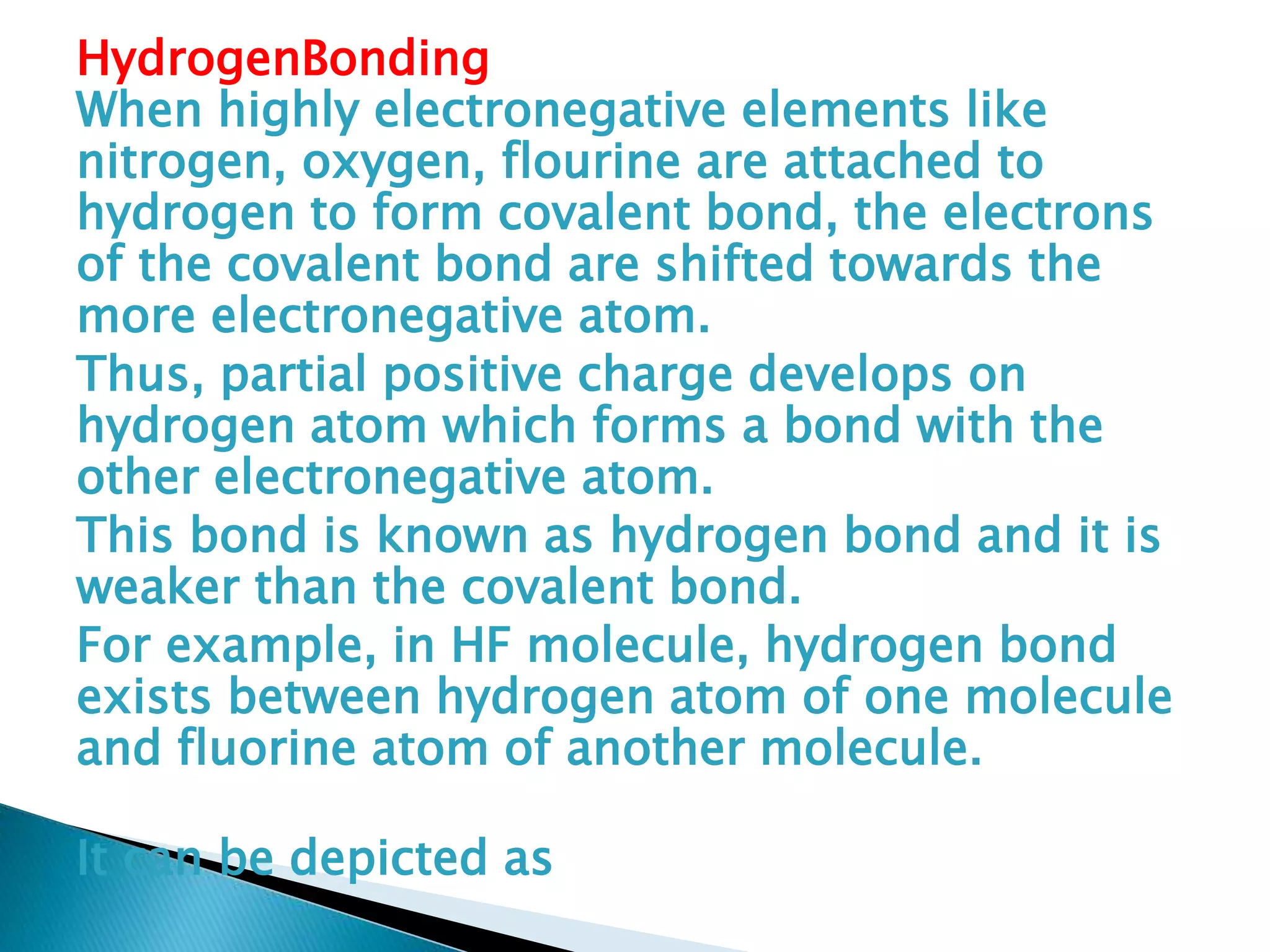
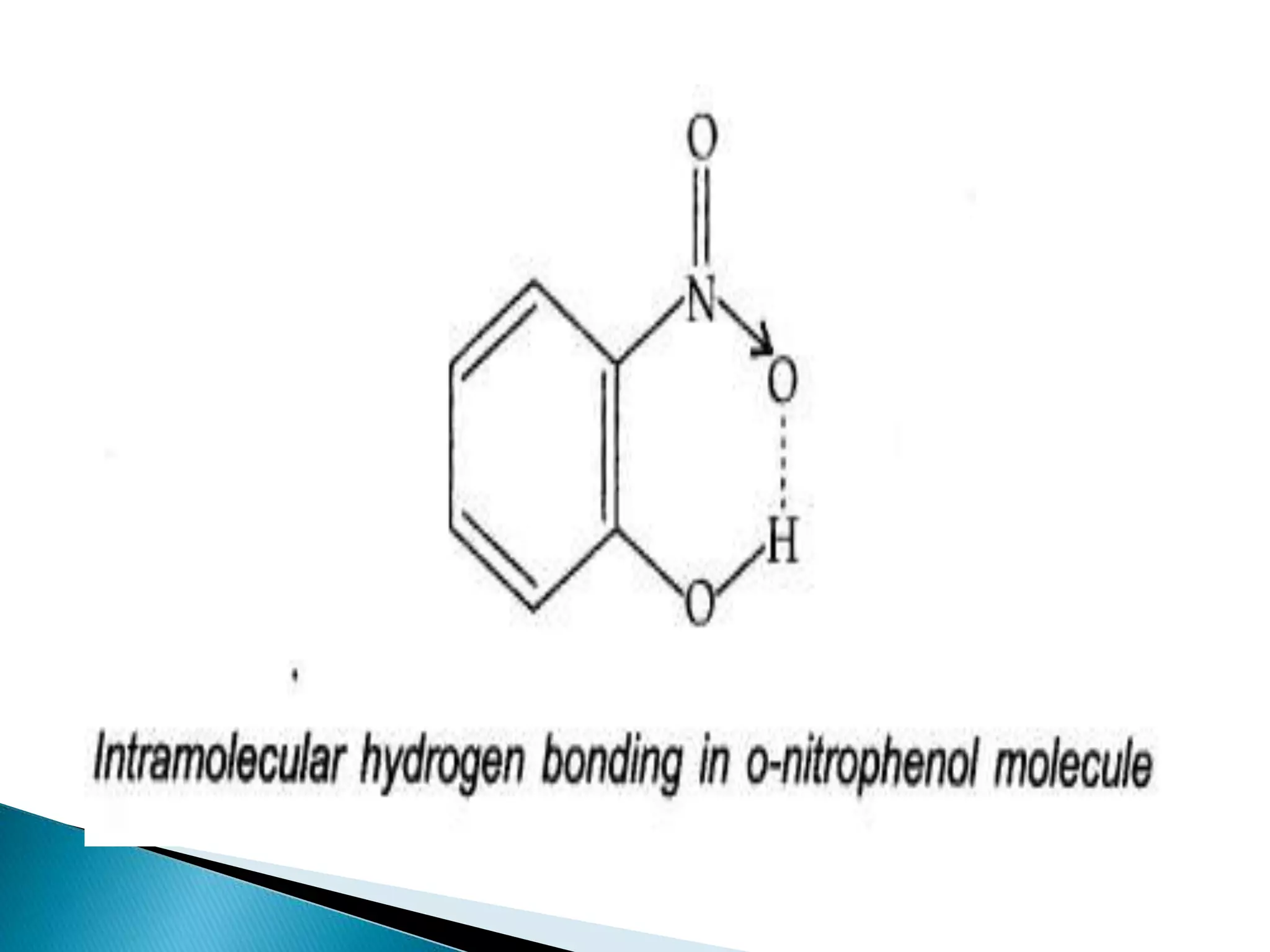
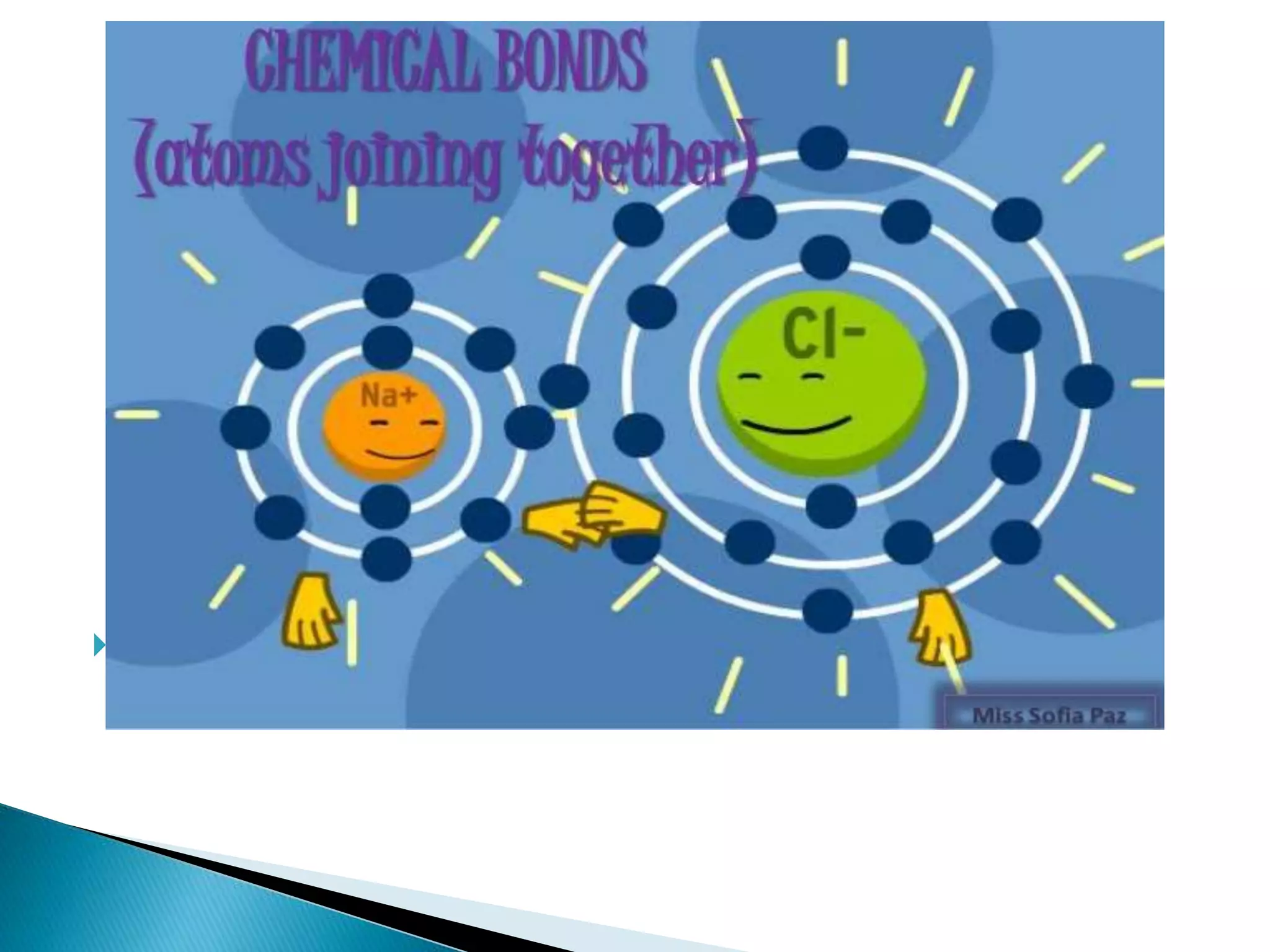
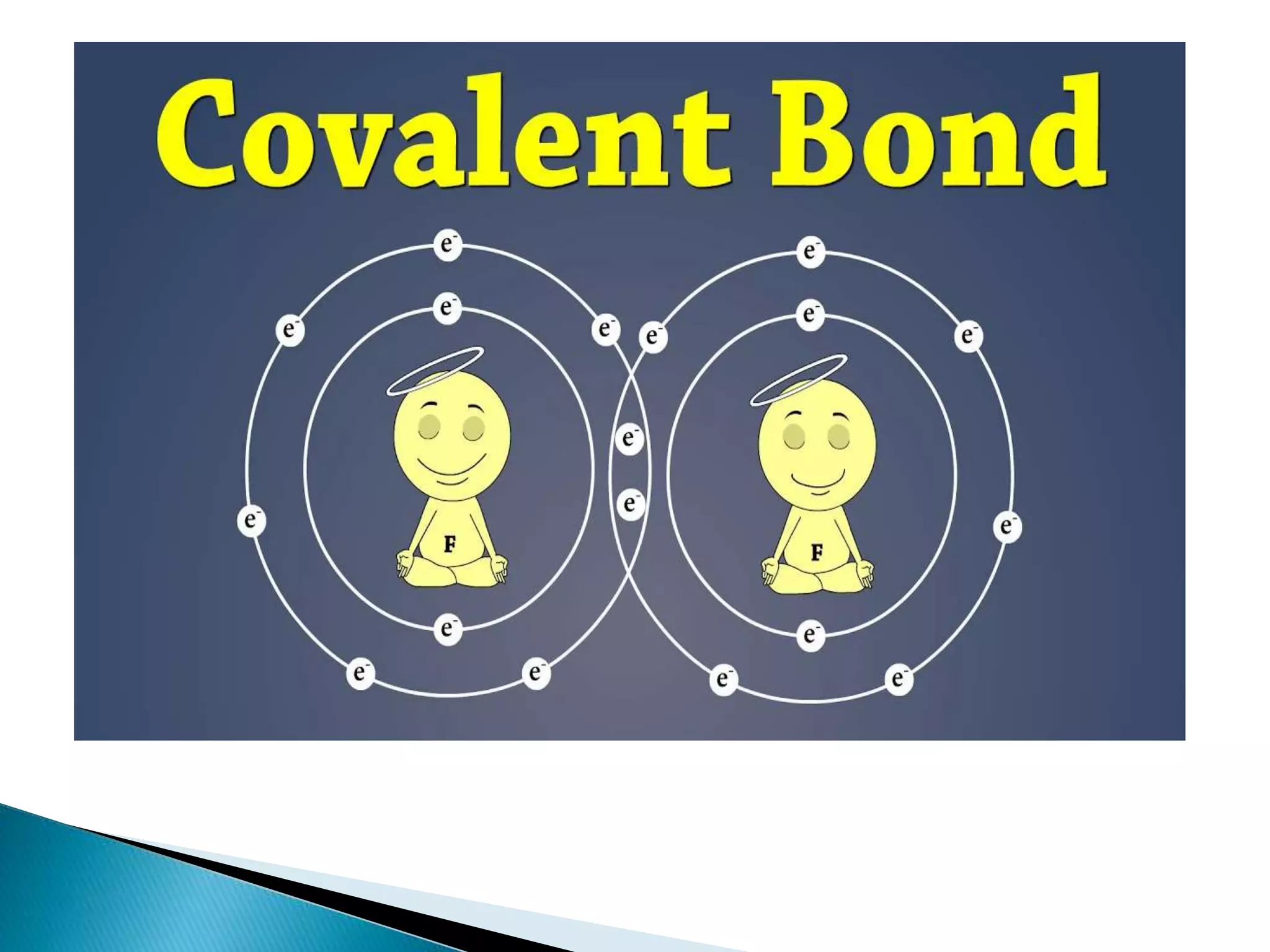
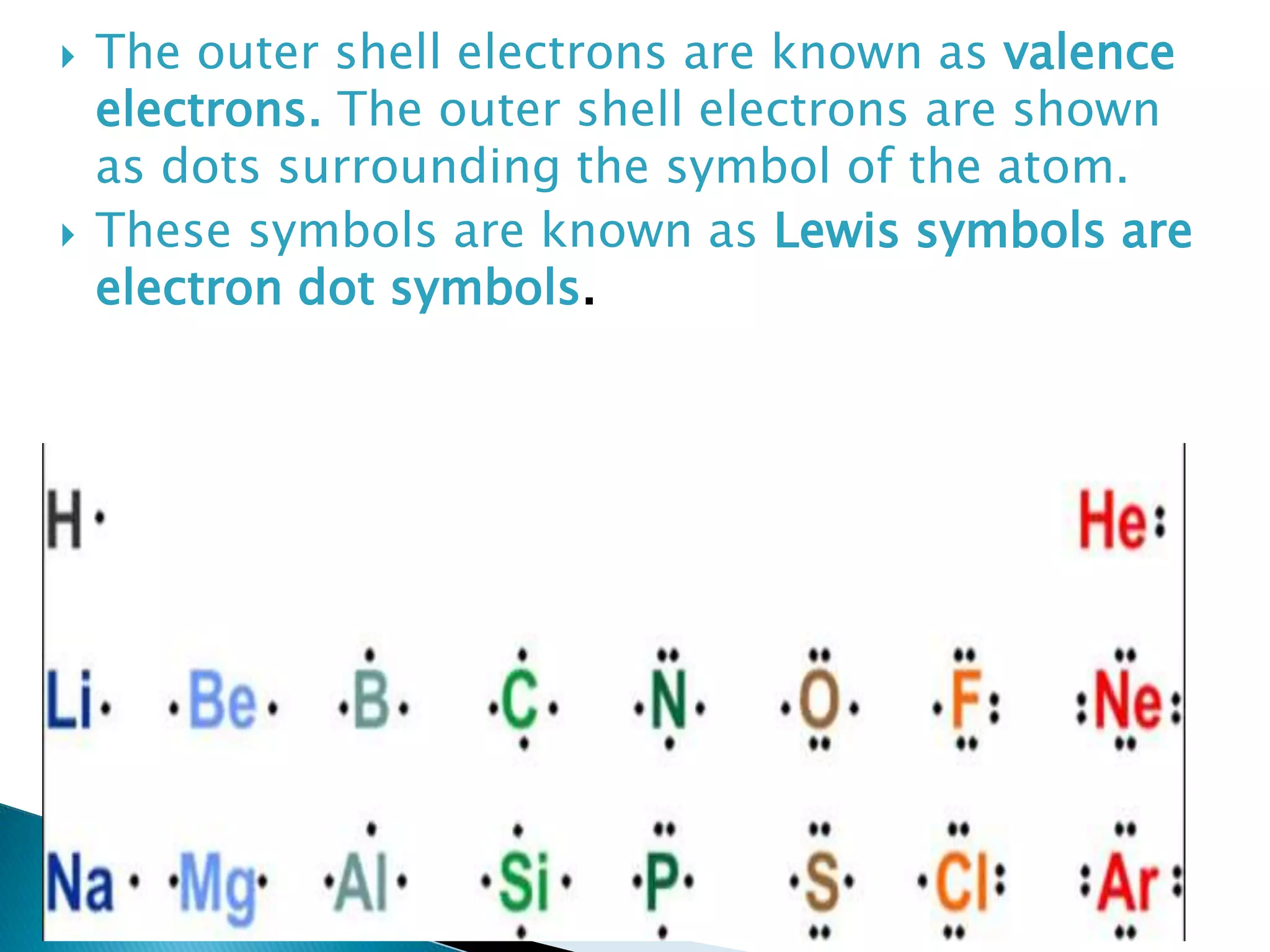
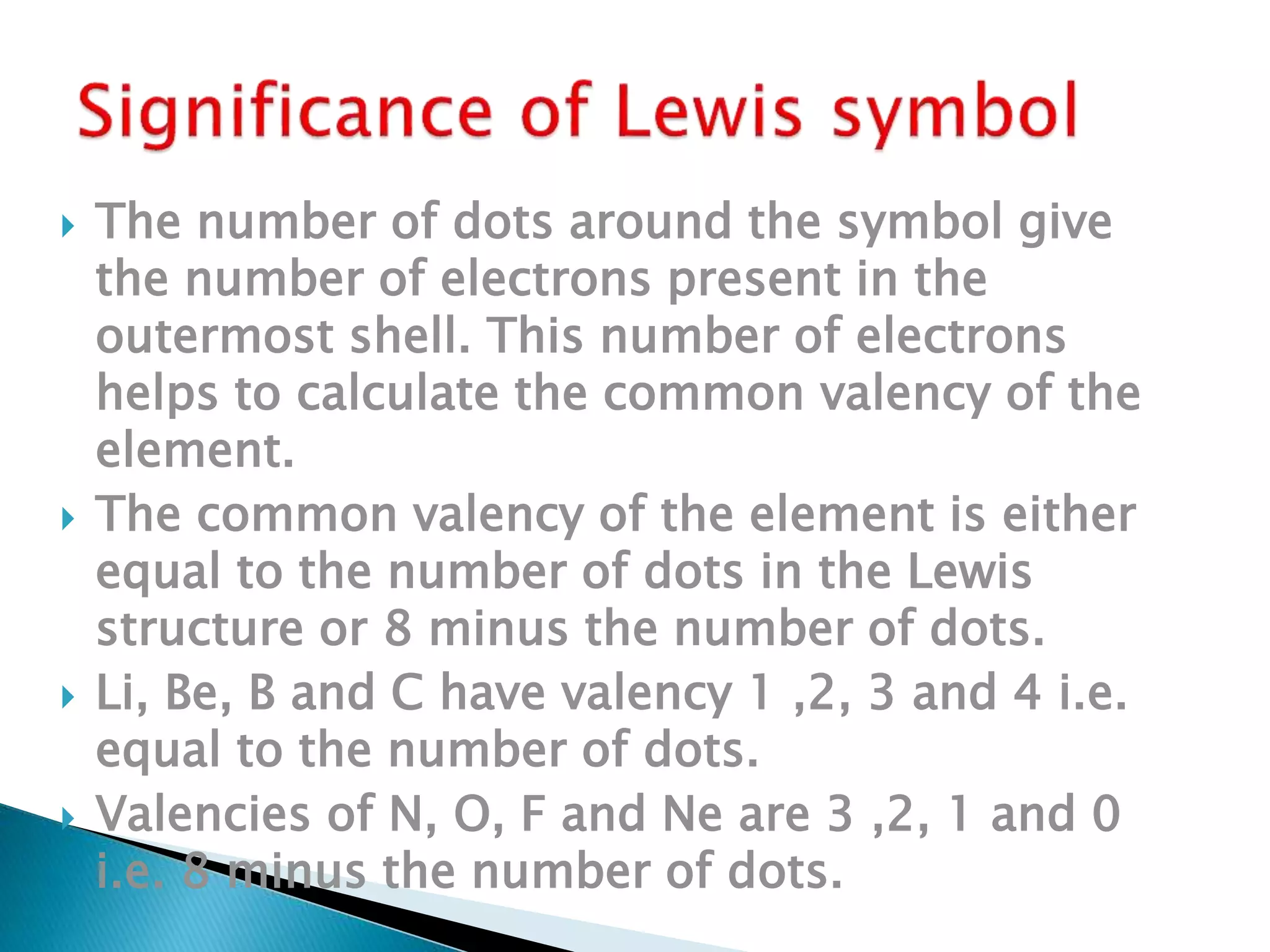
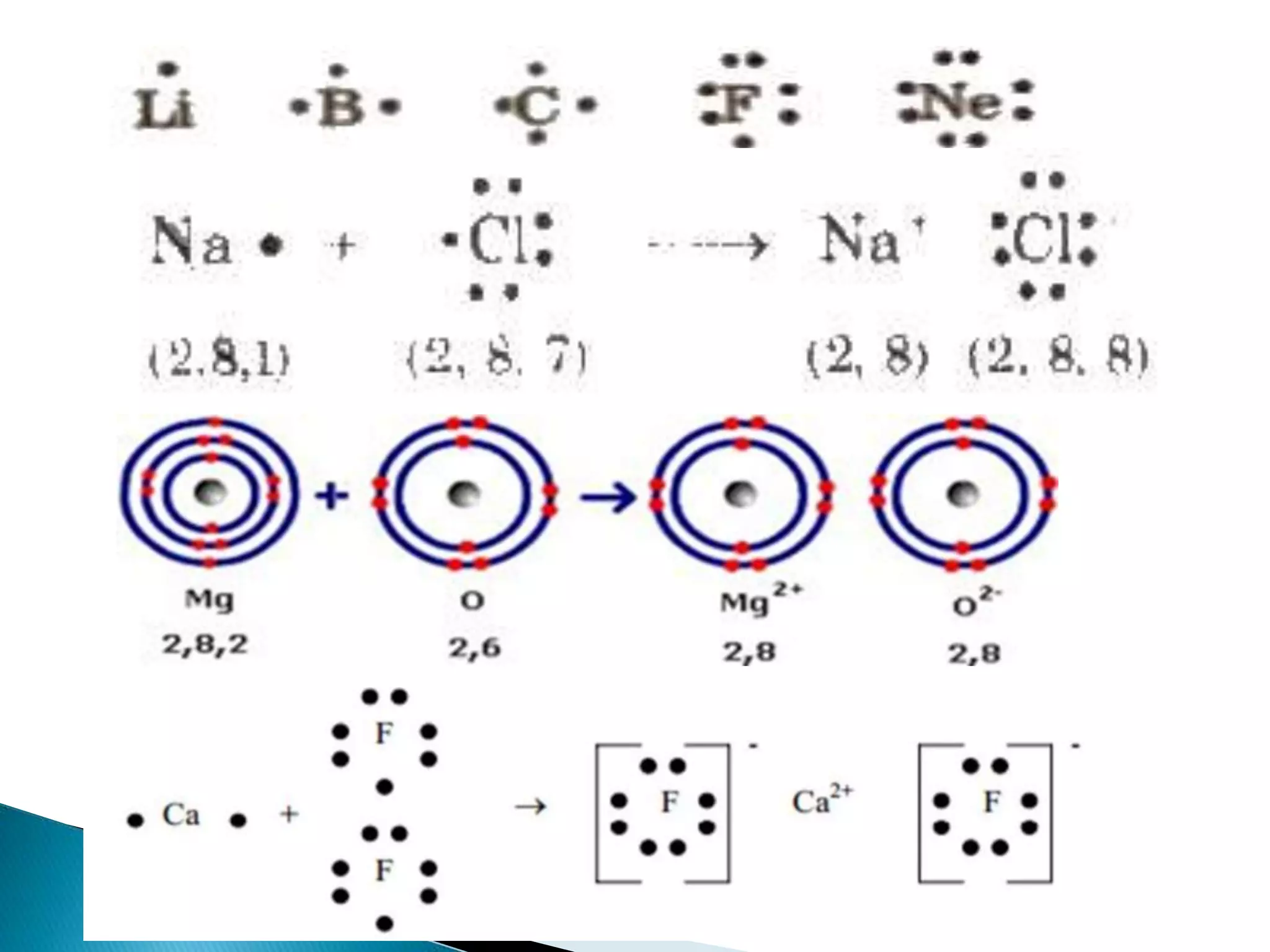
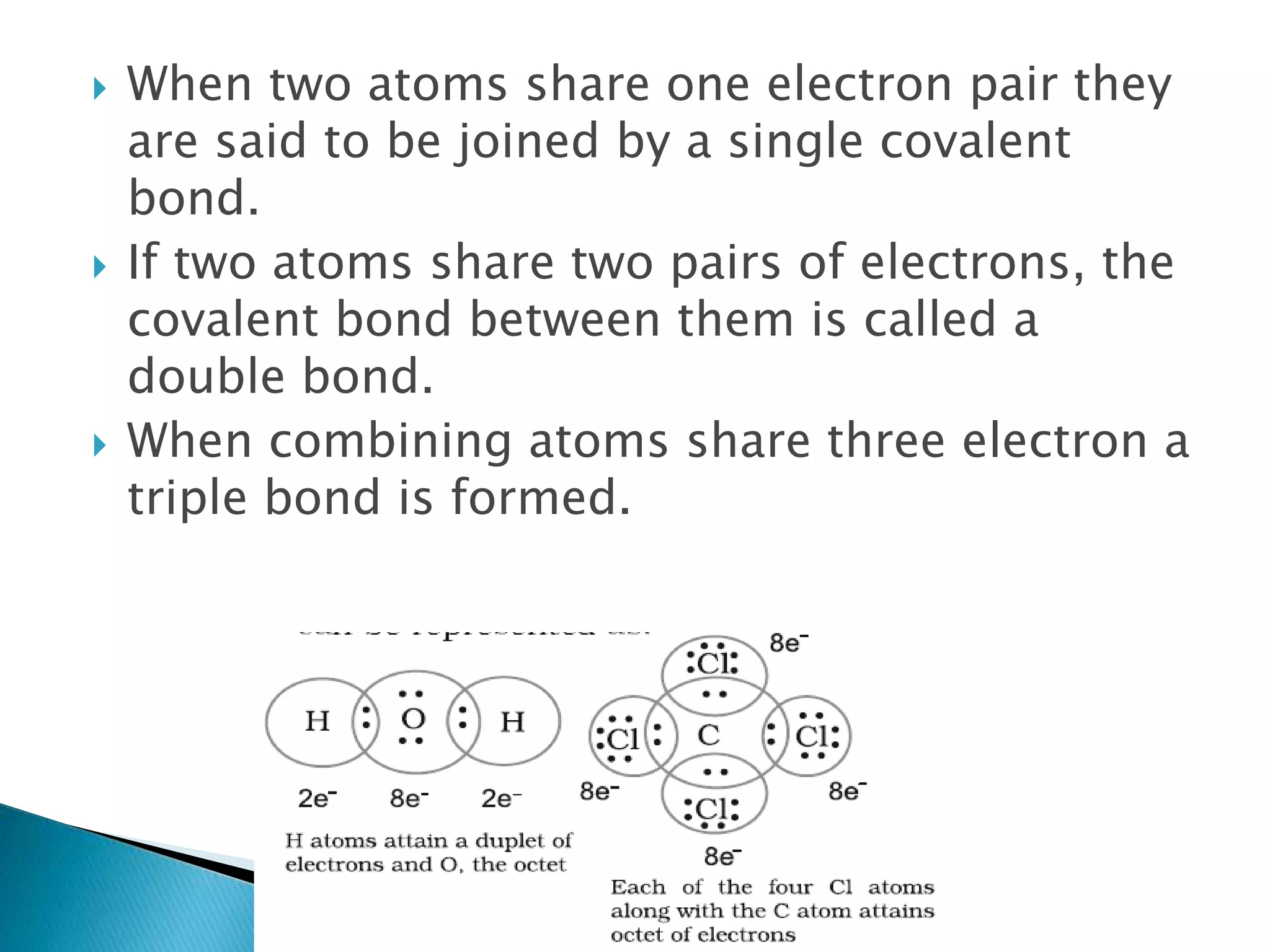
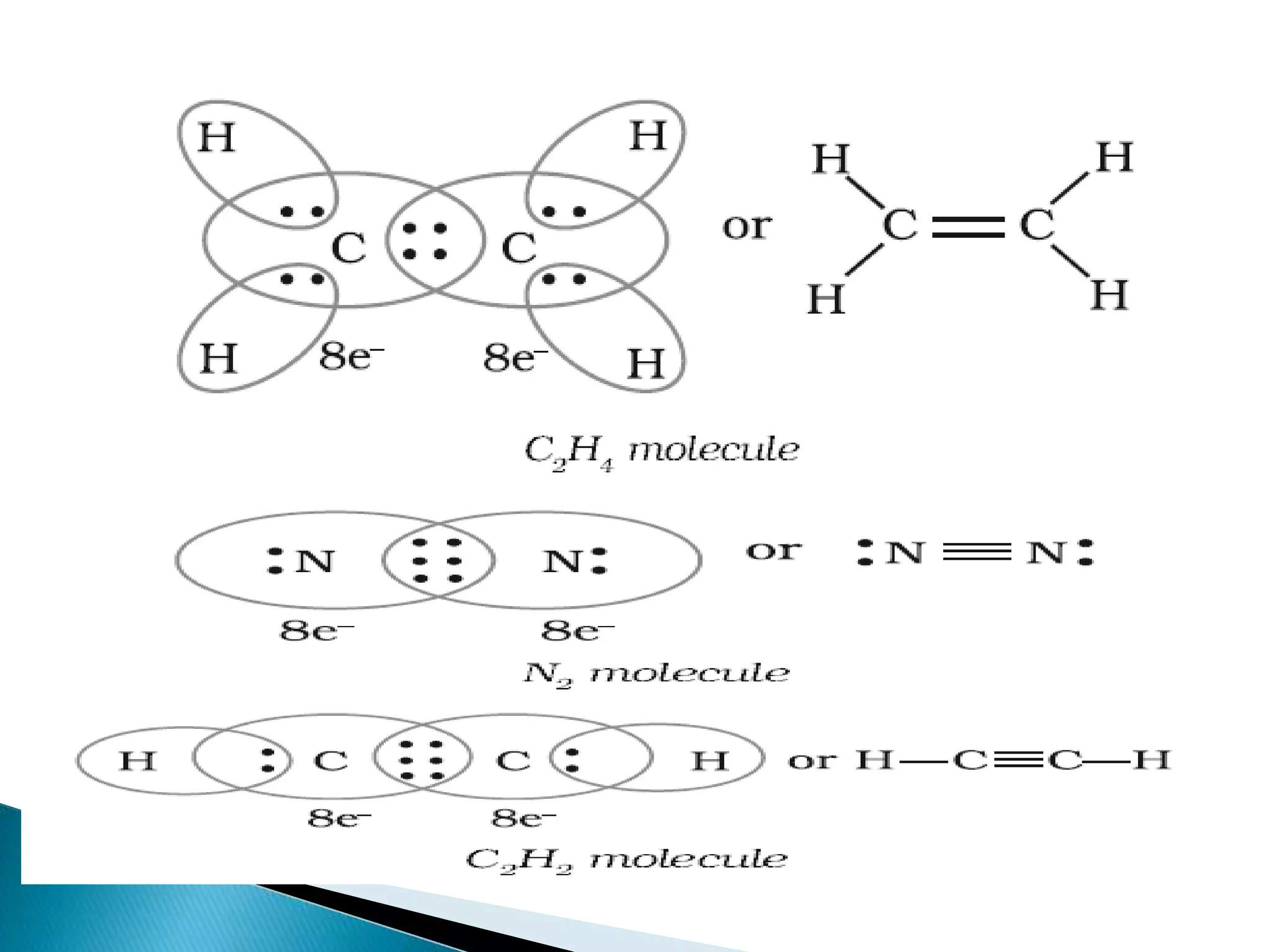
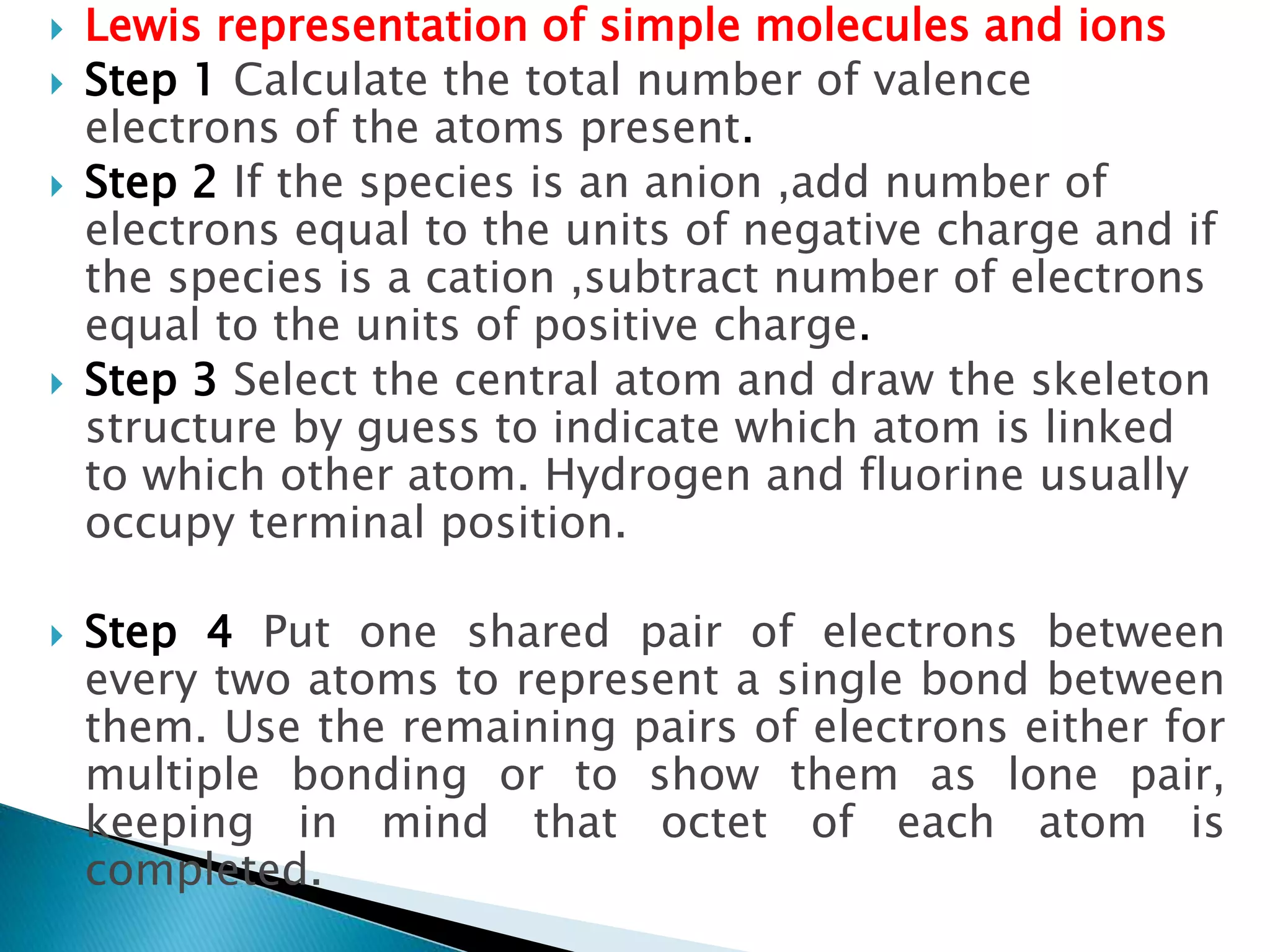
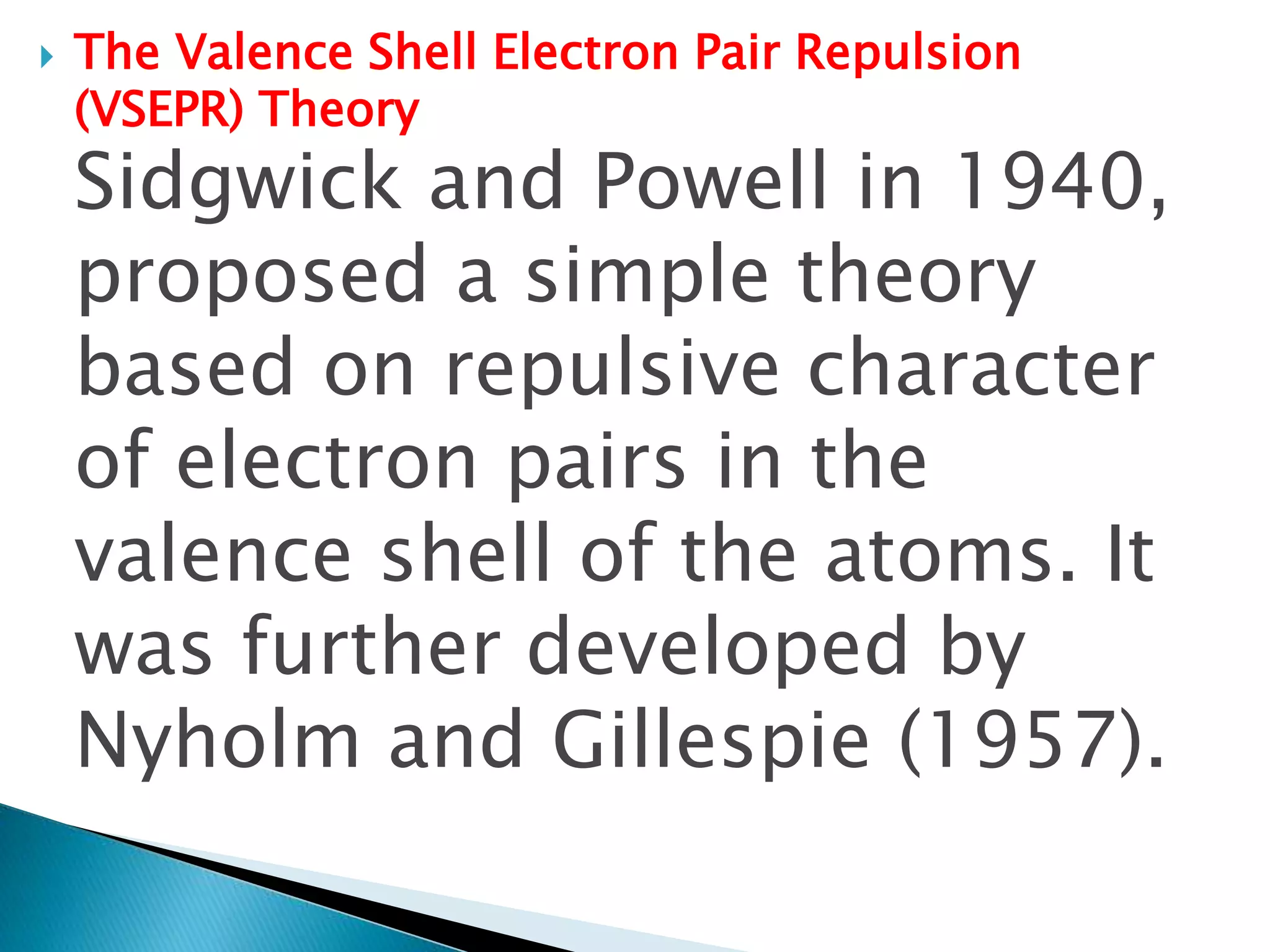
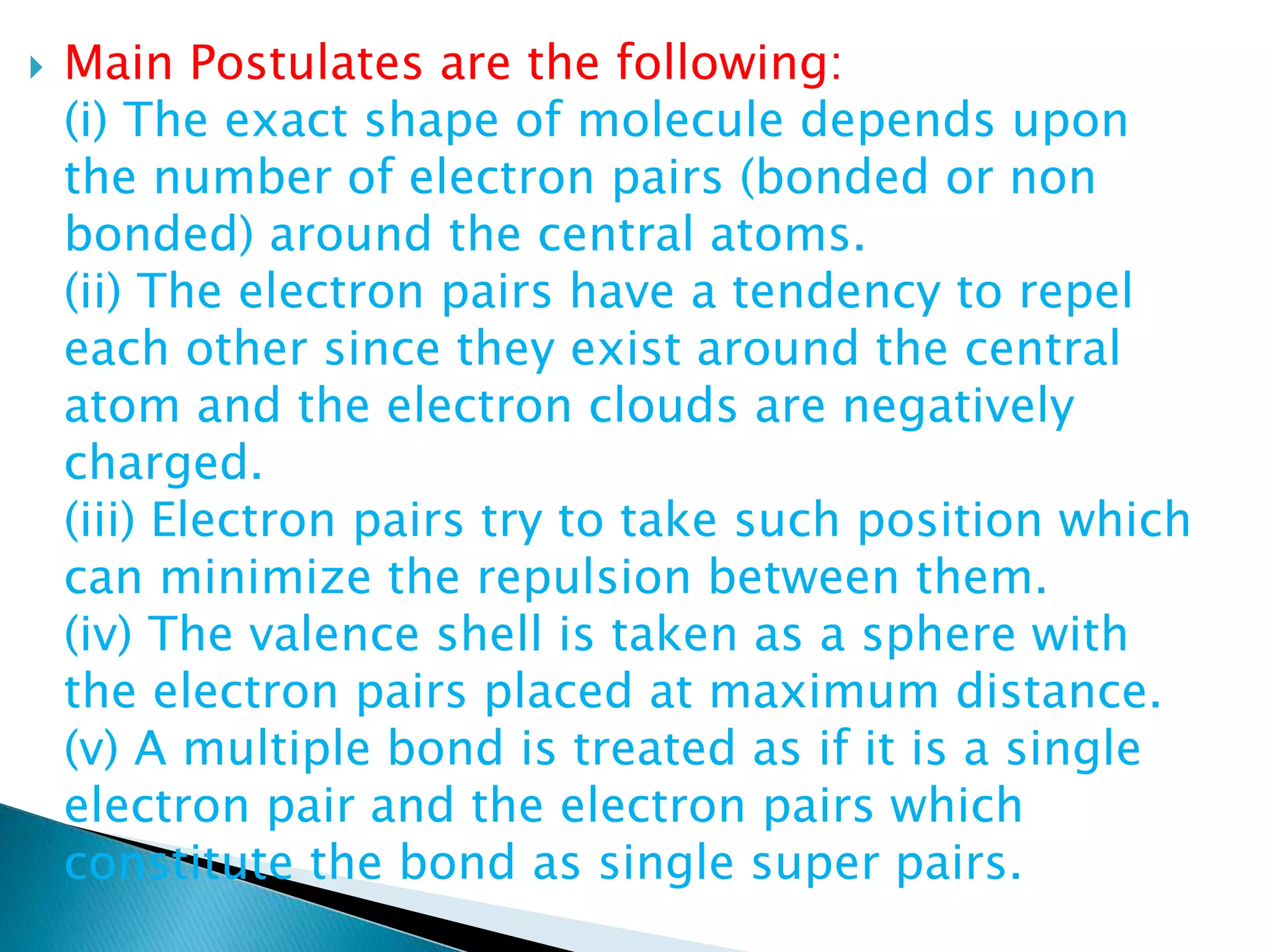
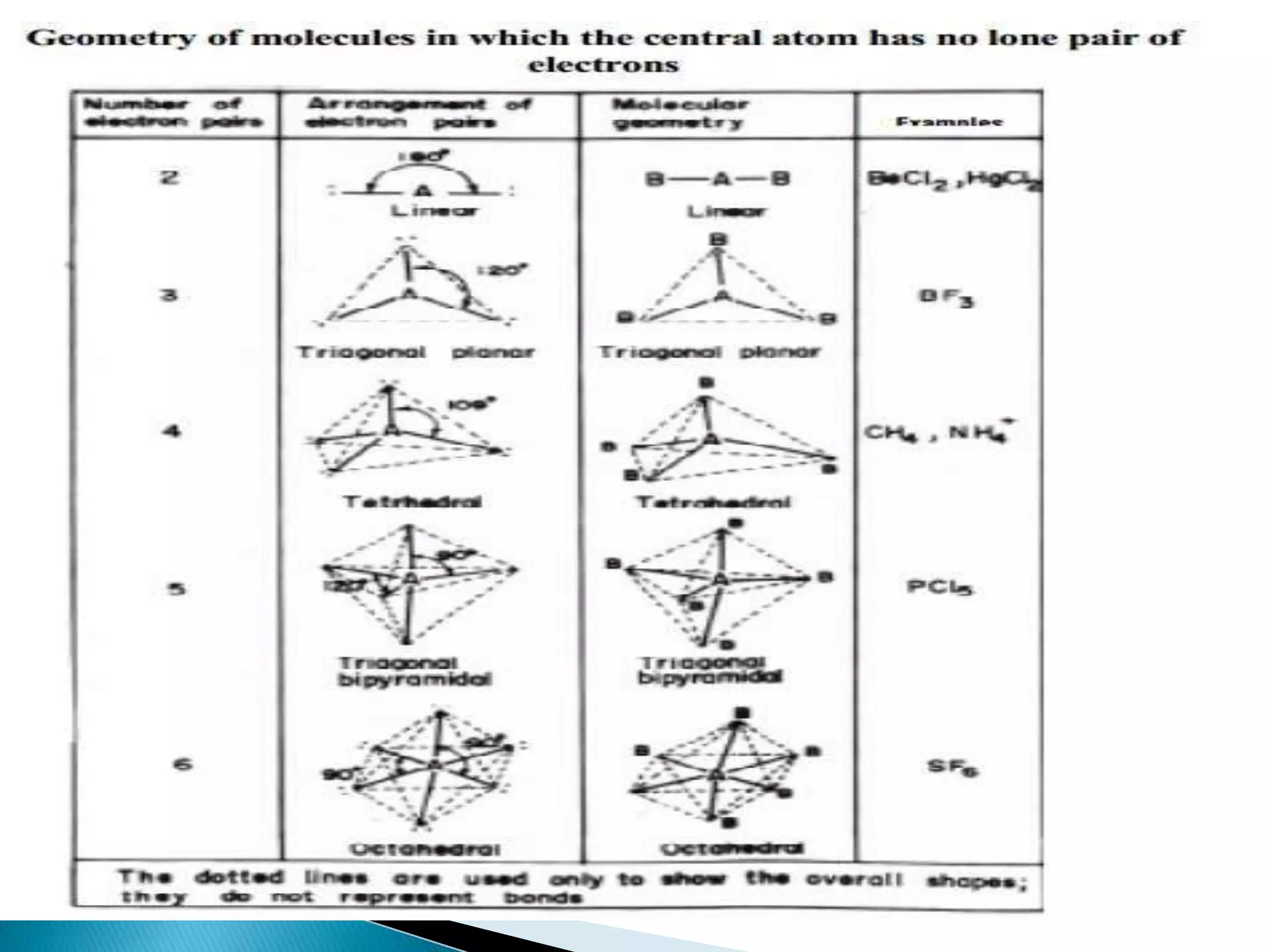
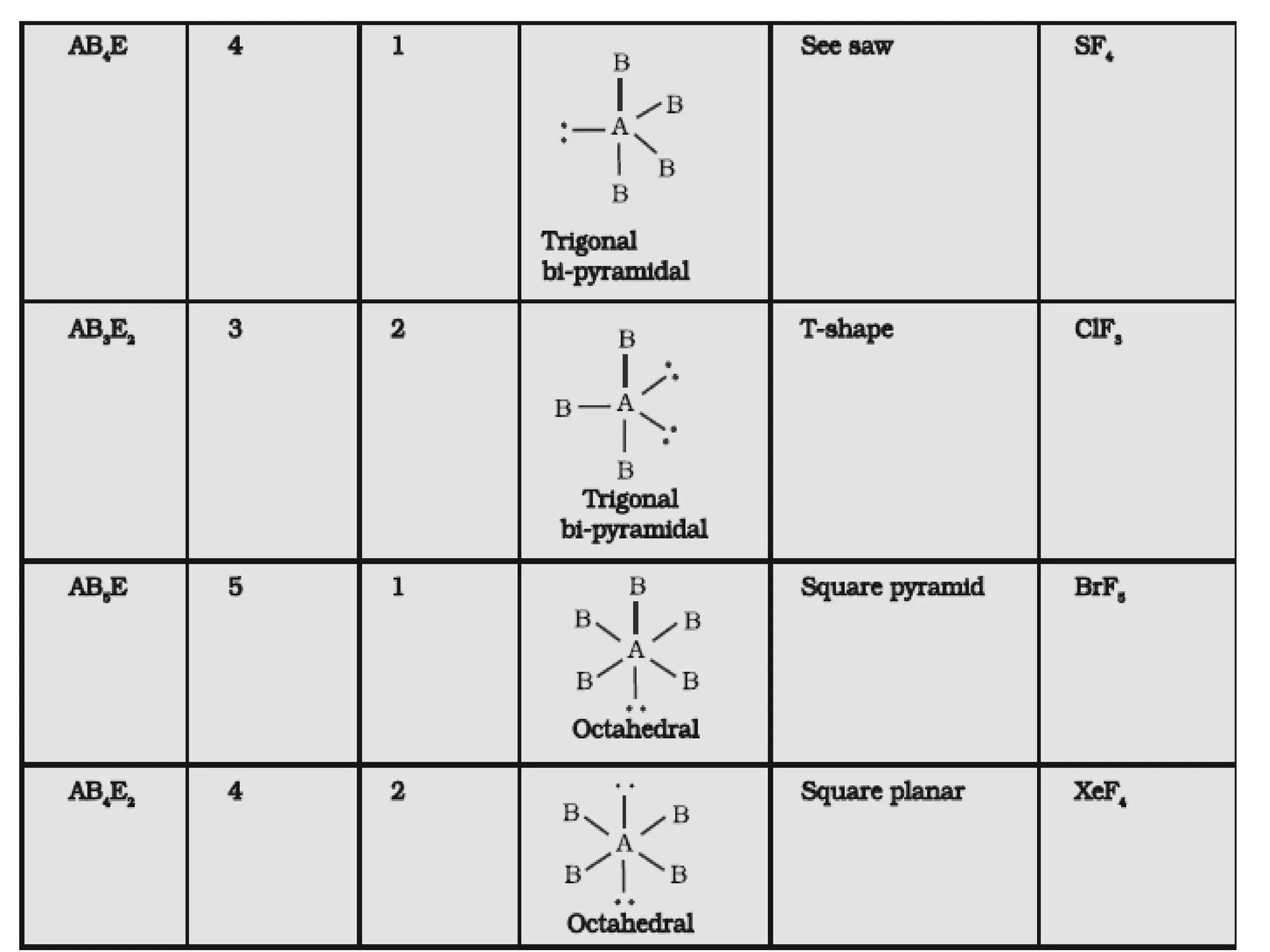
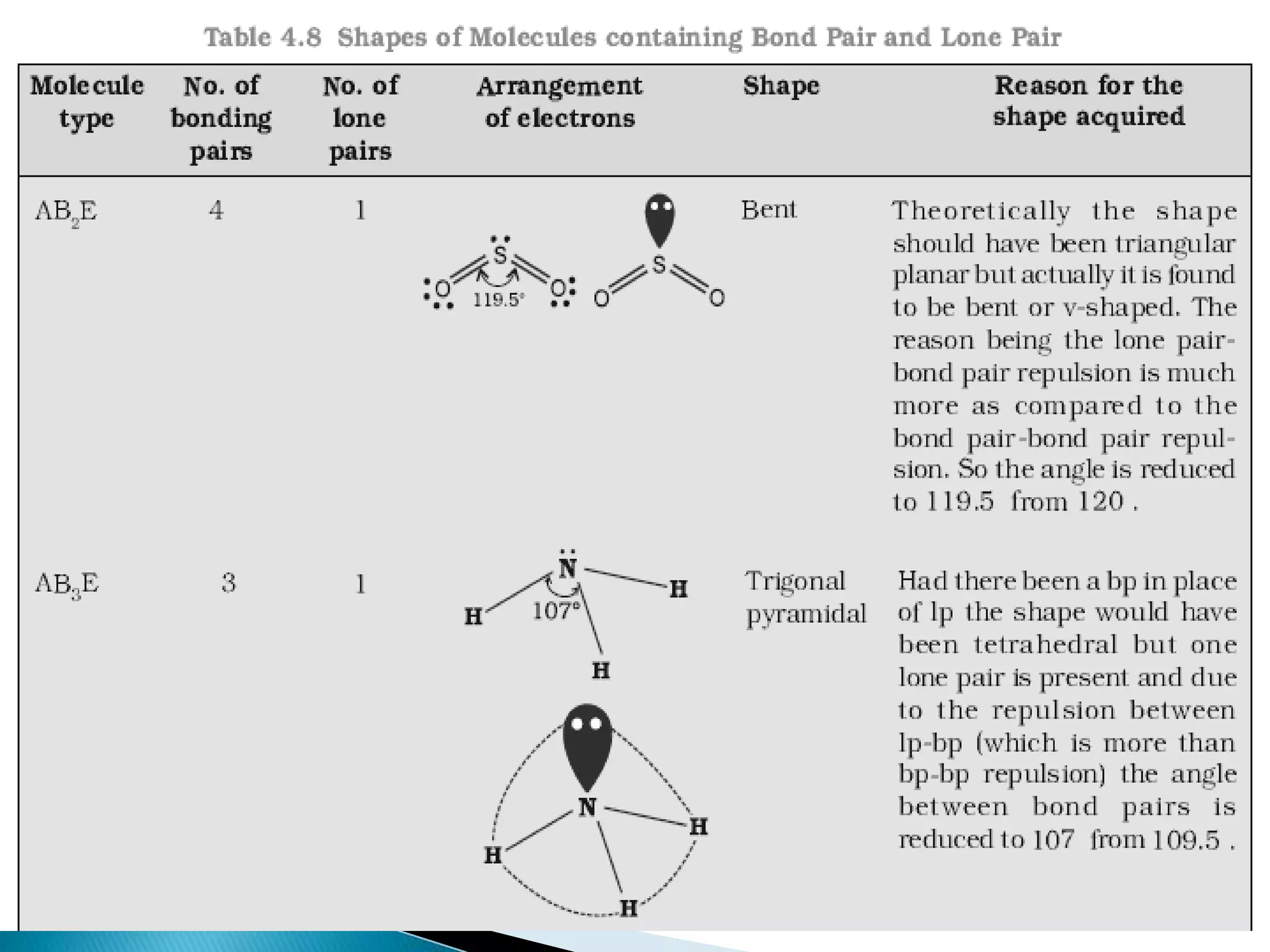
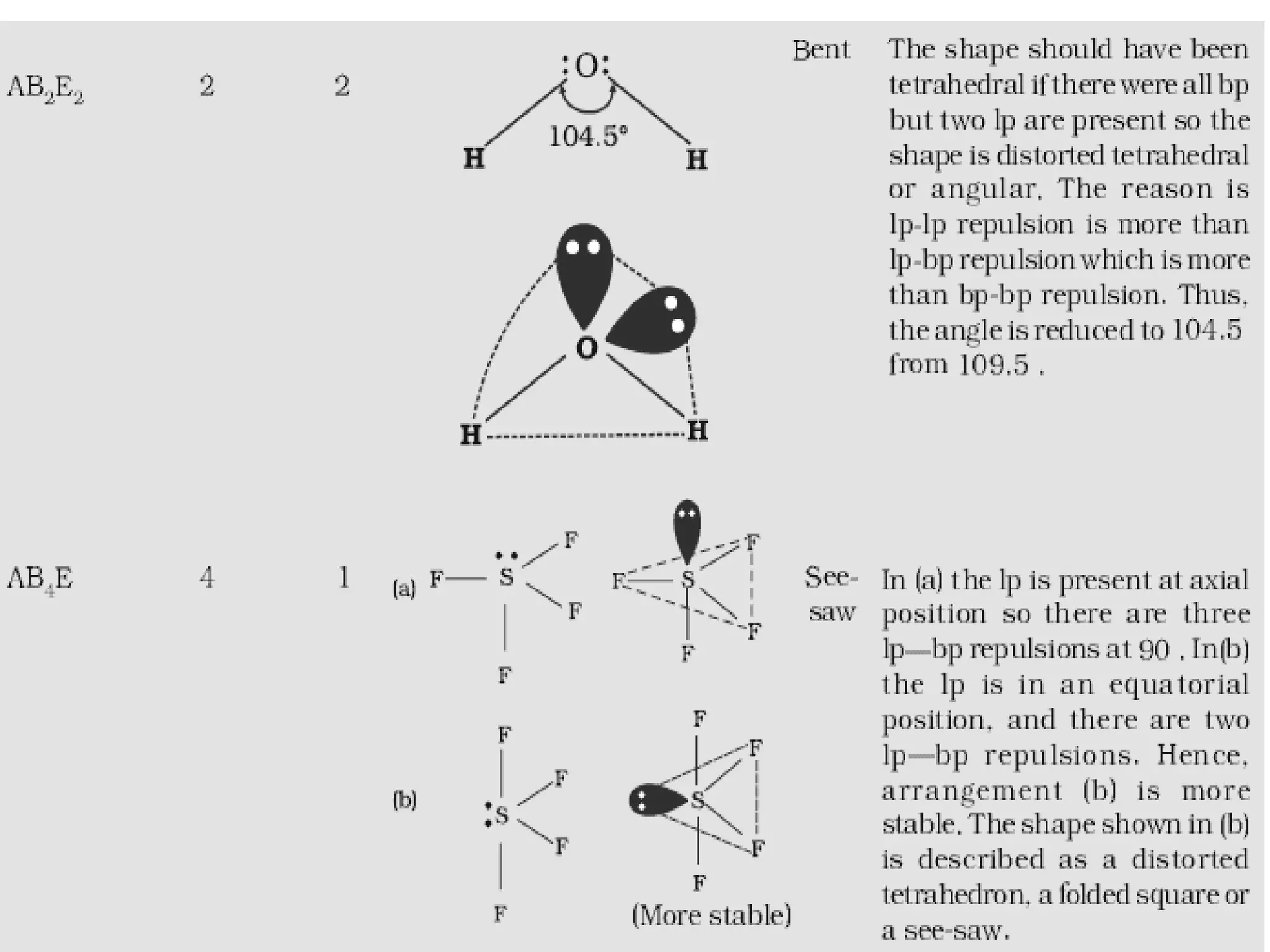
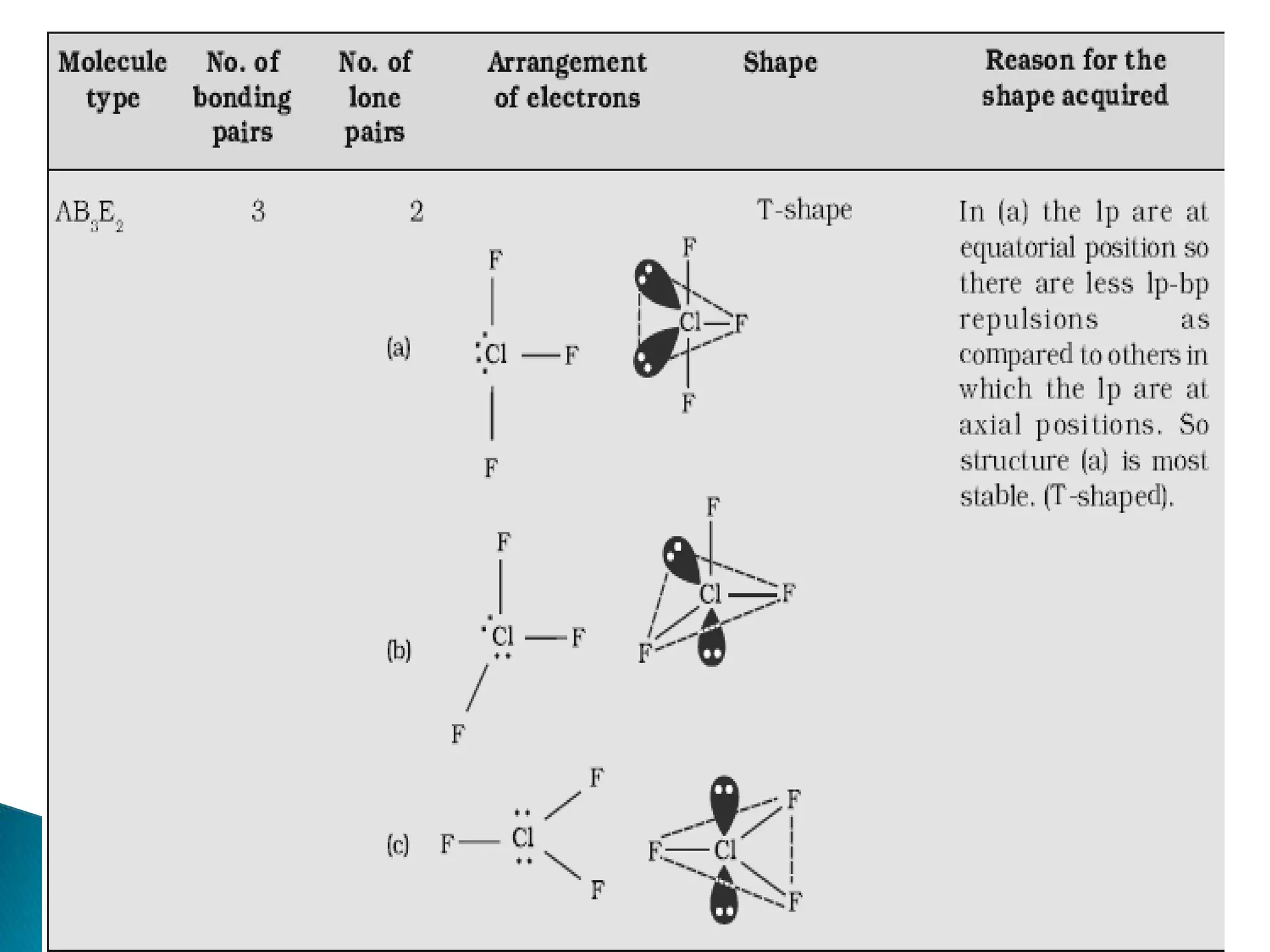
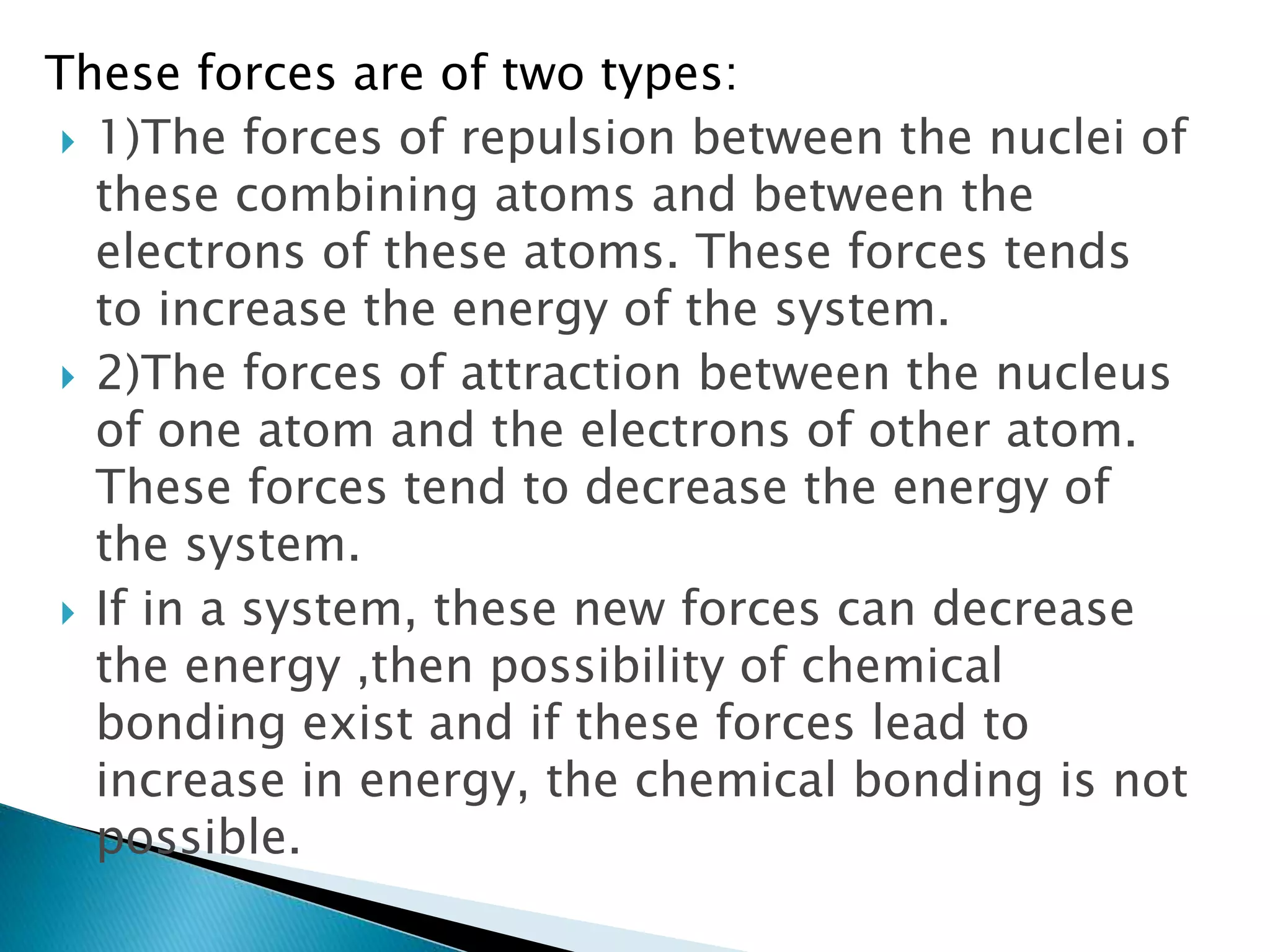
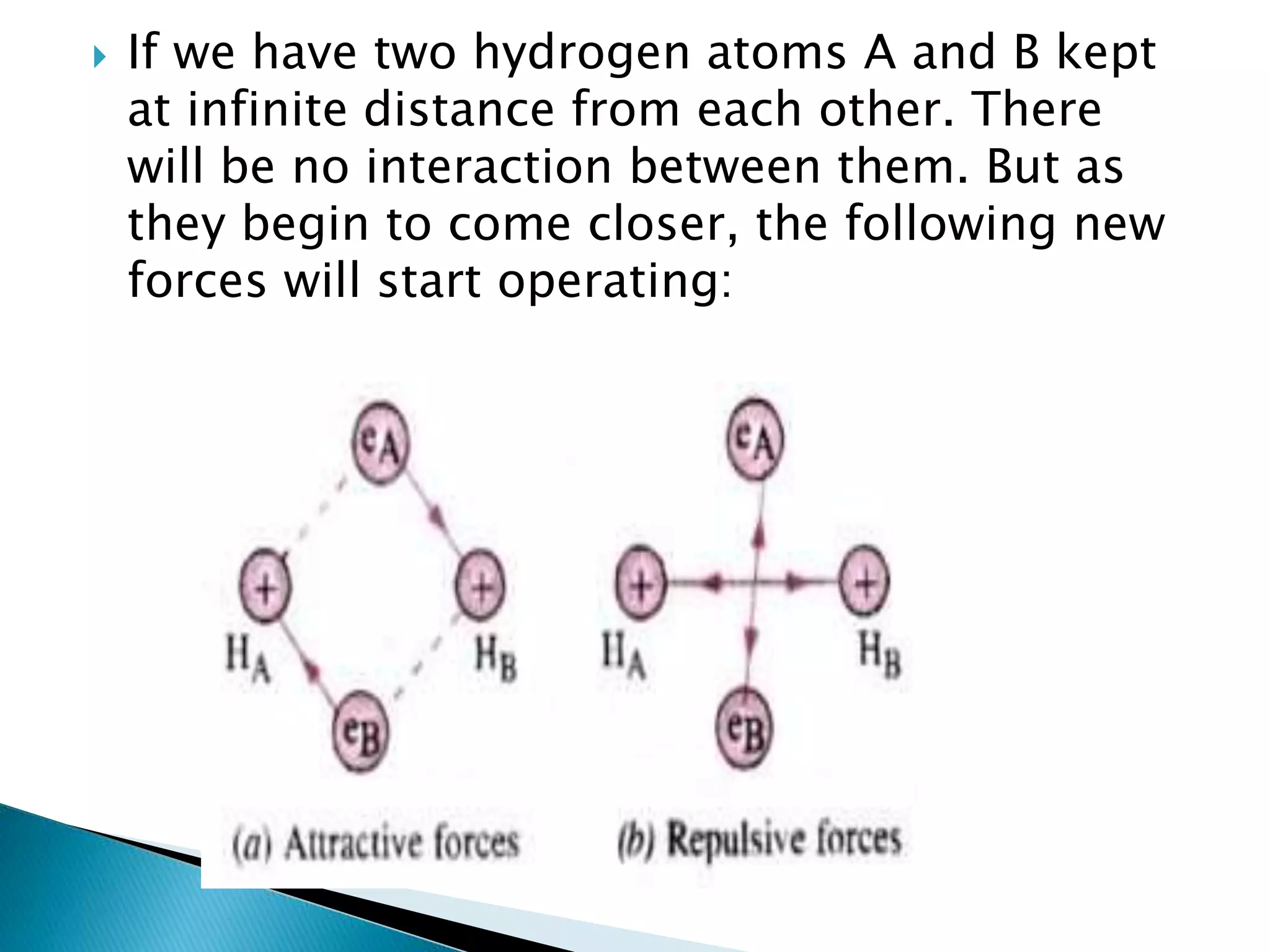
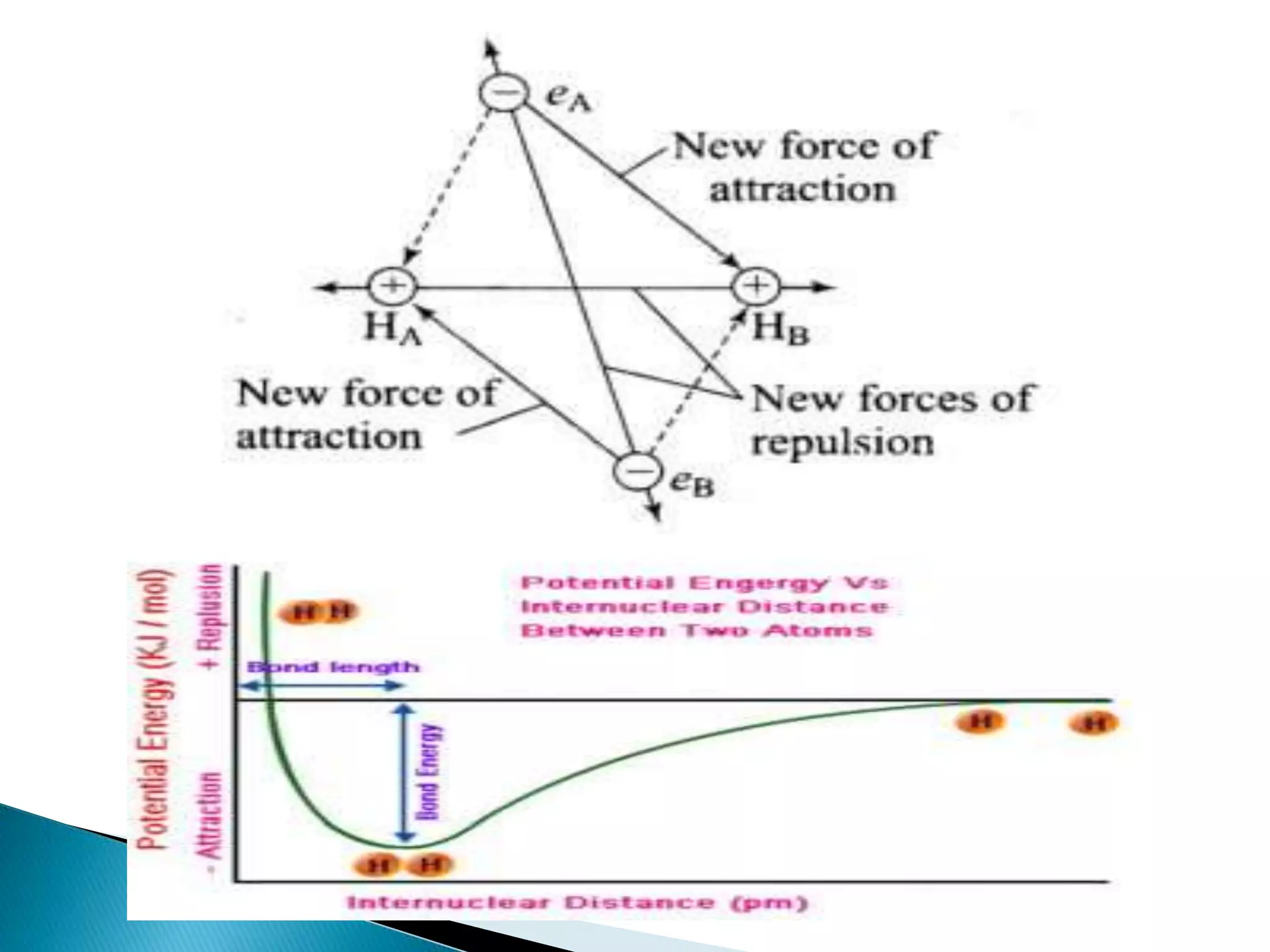
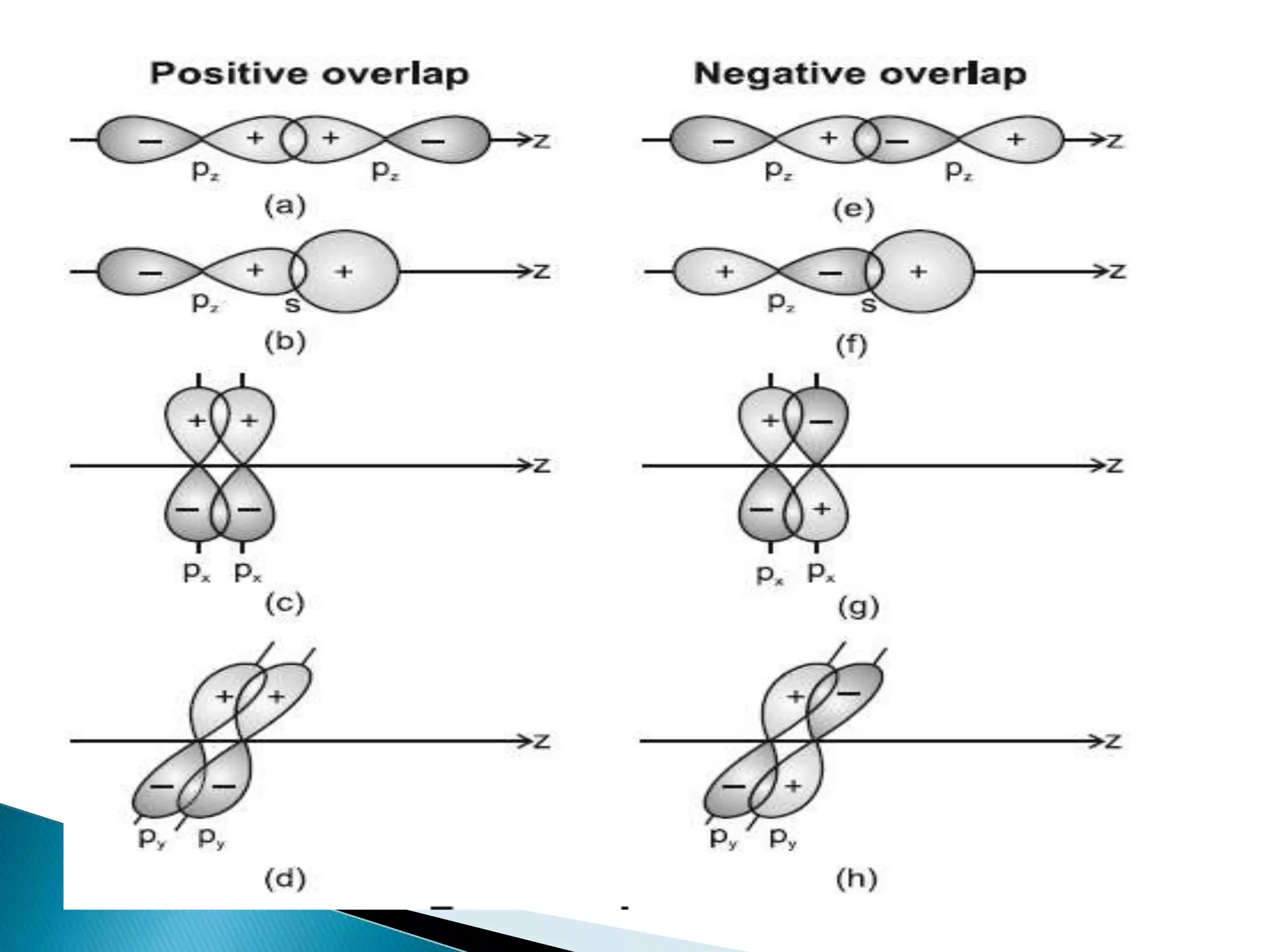
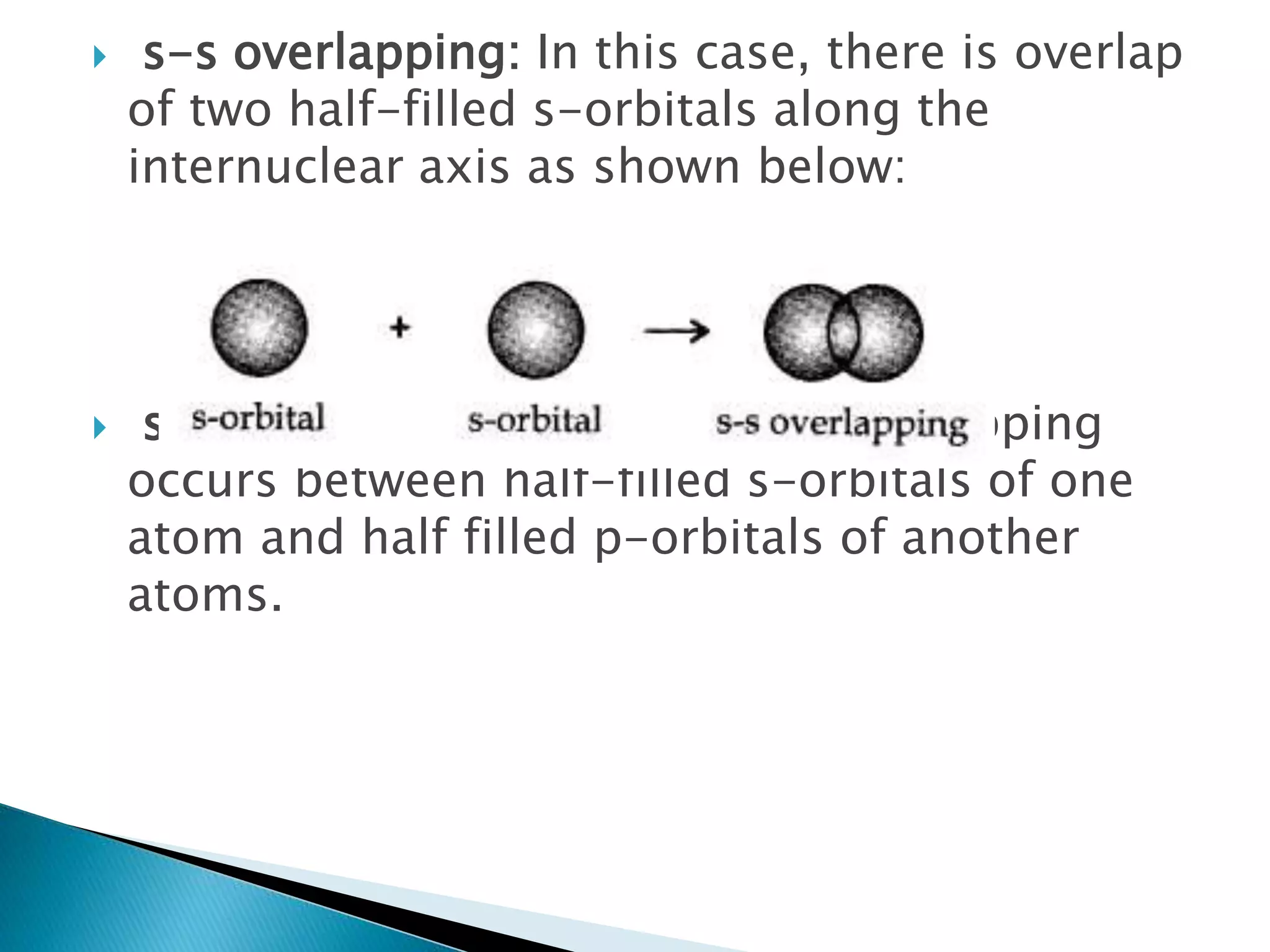
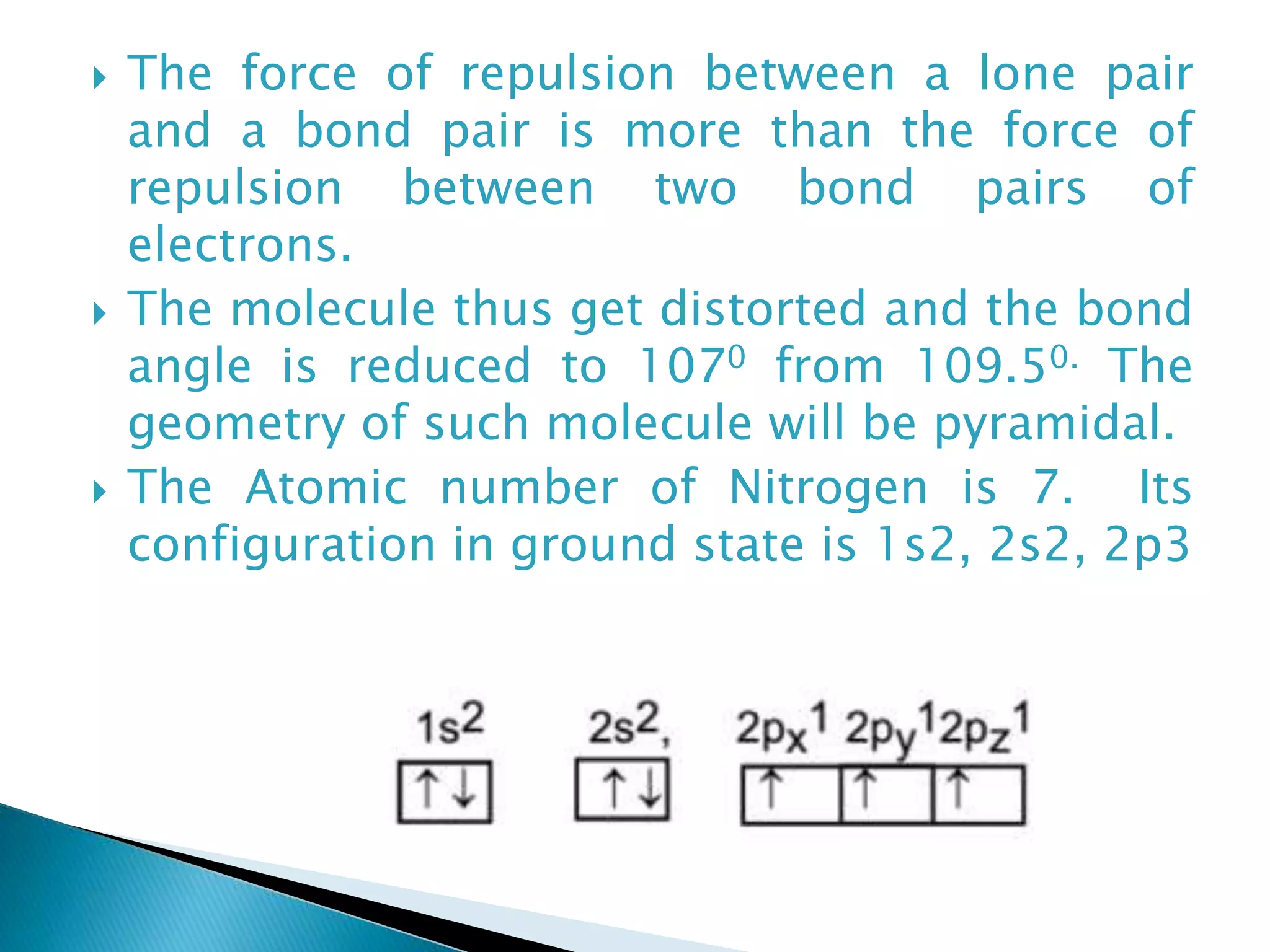
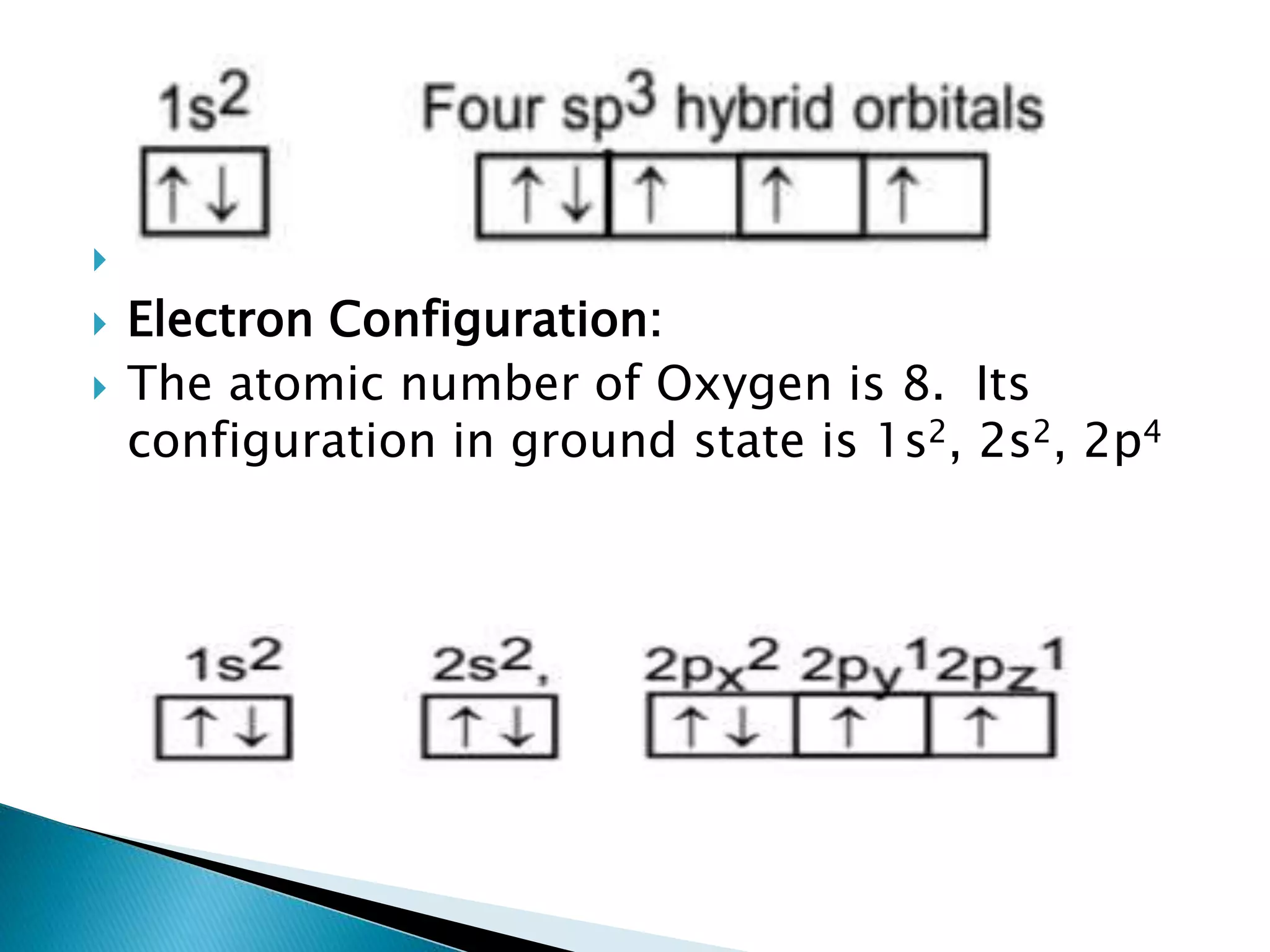
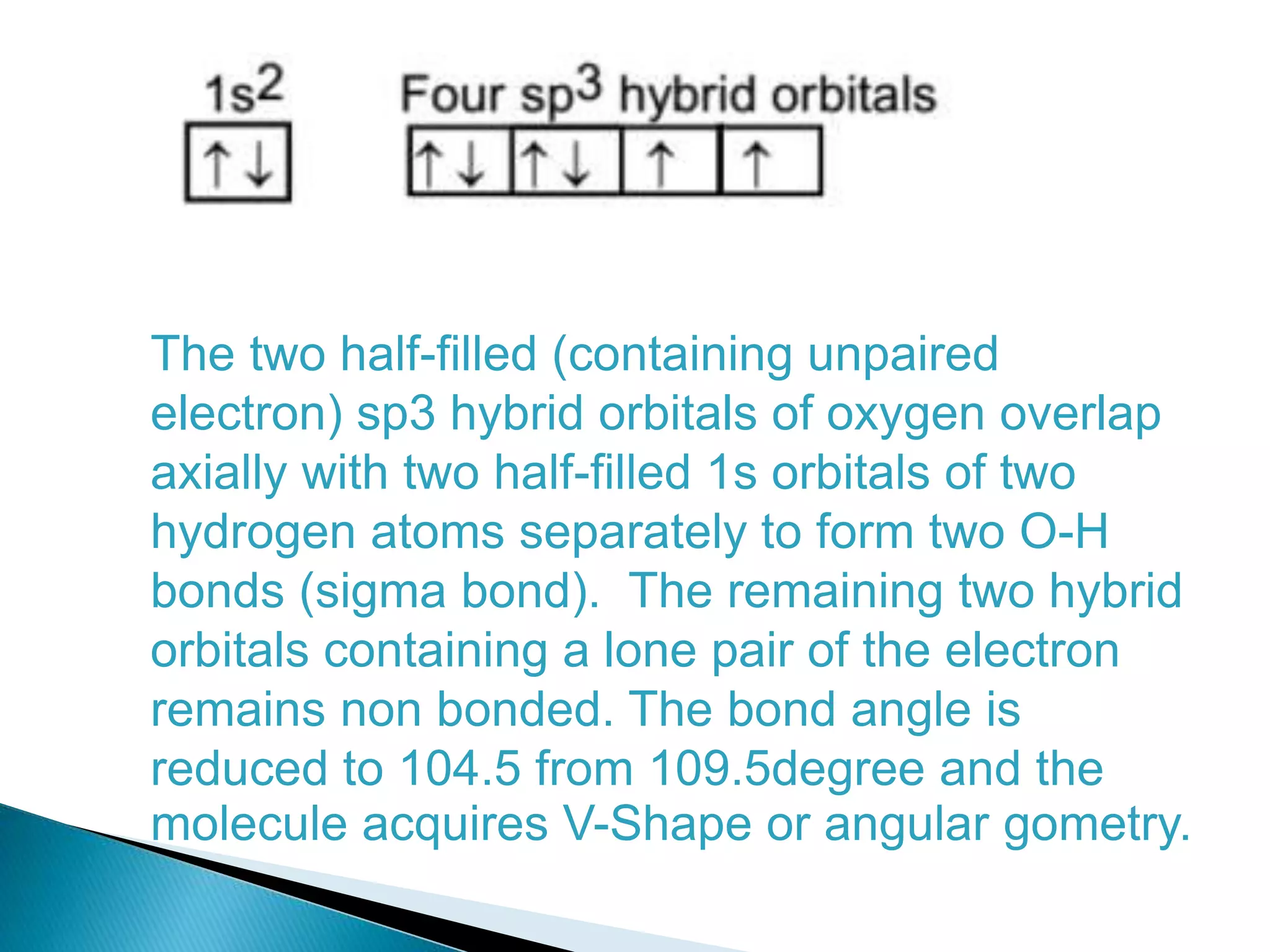
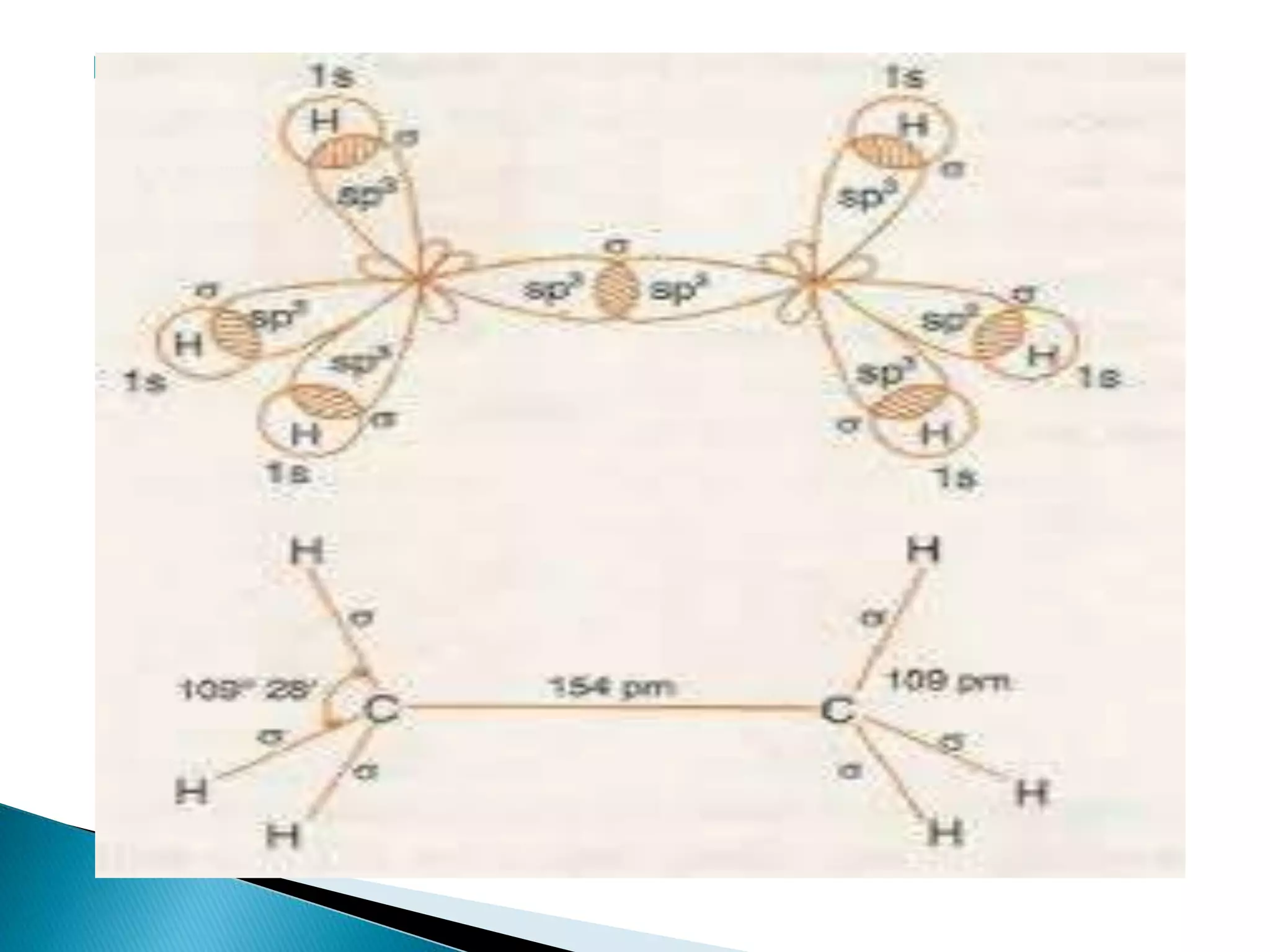
The document discusses the fundamentals of chemical bonding, including the types of bonds such as ionic and covalent, and theories like VSEPR and resonance. It explains the role of valence electrons, octet rule, and Lewis structures in determining molecular geometry and properties. Key concepts include bond enthalpy, polarity of bonds, dipole moments, and the factors influencing ionic and covalent character in compounds.




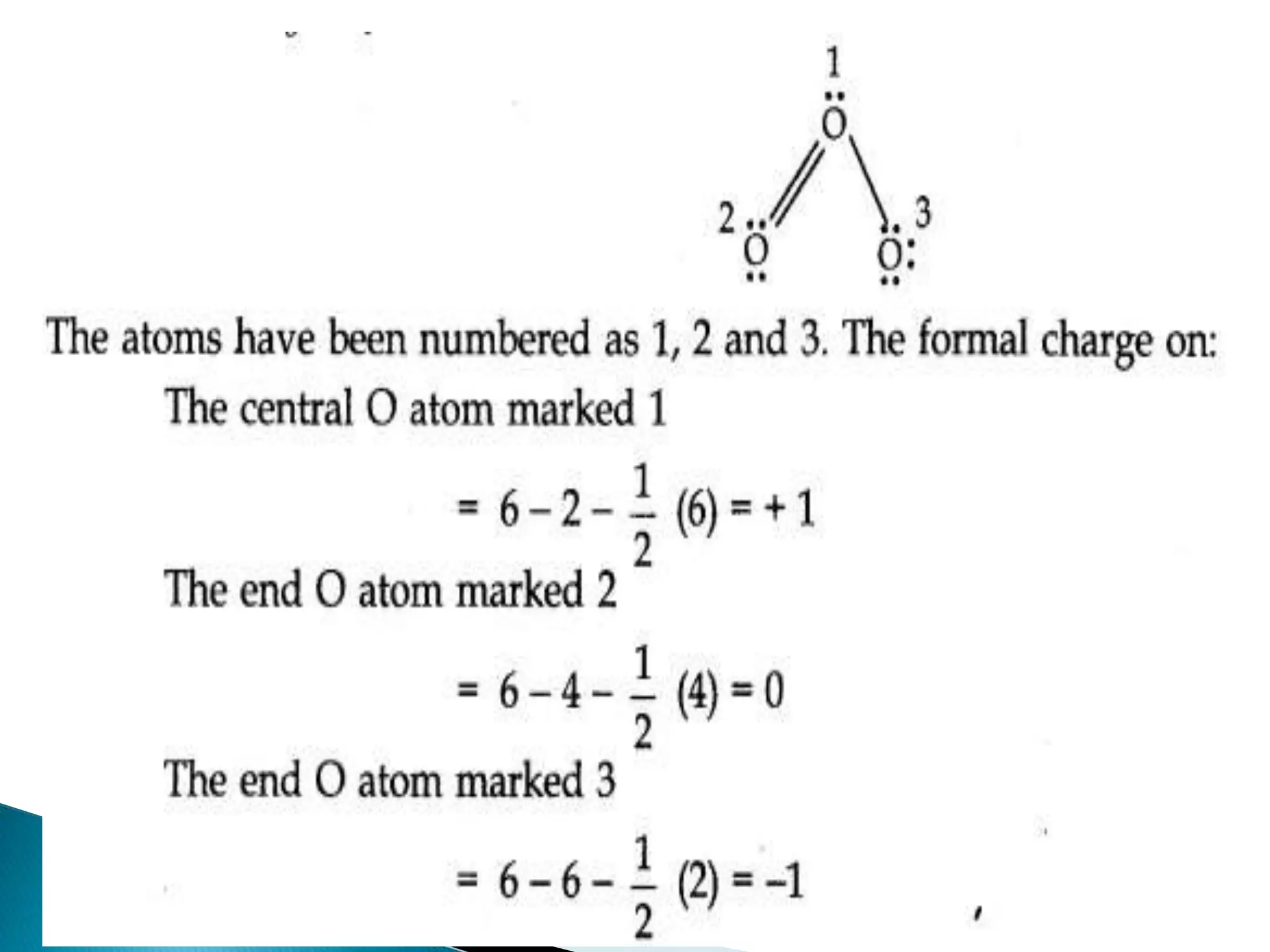
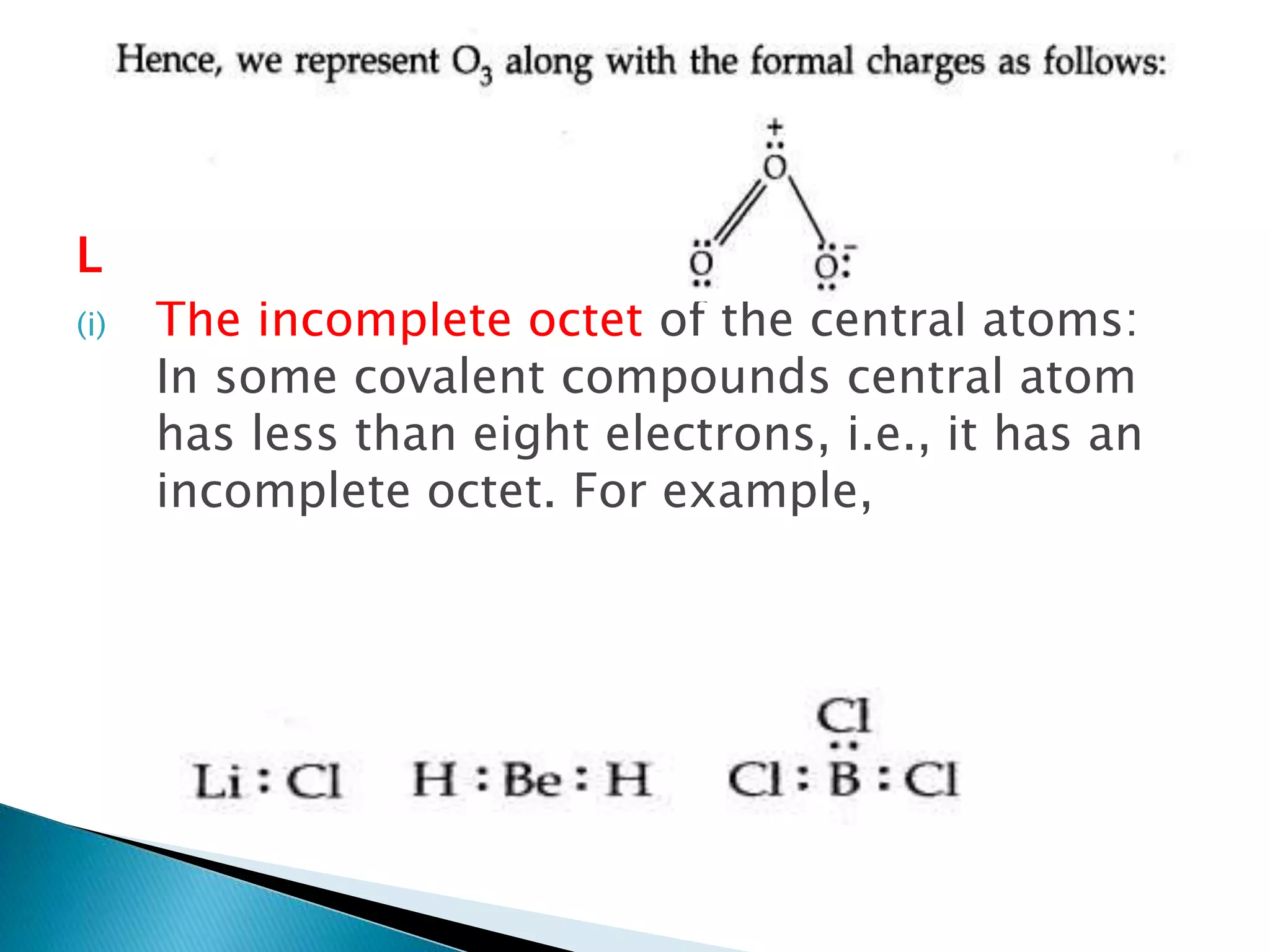
![ Formal Charge:
In polyatomic ions, the net charge is the charge on
the ion as a whole and not by particular atom.
However, charges can be assigned to individual
atoms or ions. These are called formal charges.
Formal charge=
Valence electrons- Non bonding electrons-
𝟏
𝟐
[𝒔𝒉𝒂𝒓𝒆𝒅 𝒆𝒍𝒆𝒄𝒕𝒓𝒐𝒏𝒔]](https://image.slidesharecdn.com/cbms-200921151134/75/chemical-bonding-and-molecular-structure-class-11-22-2048.jpg)




































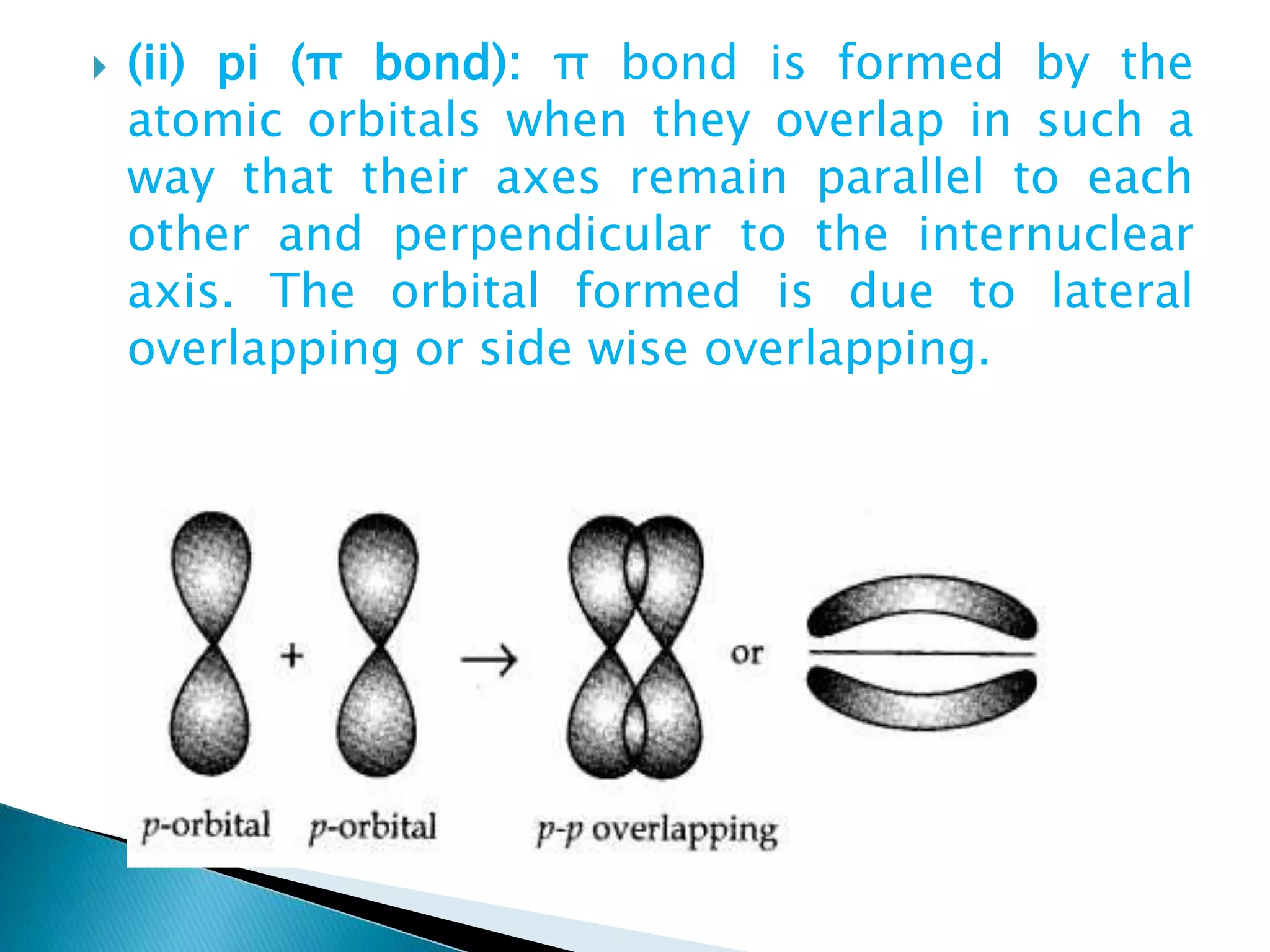










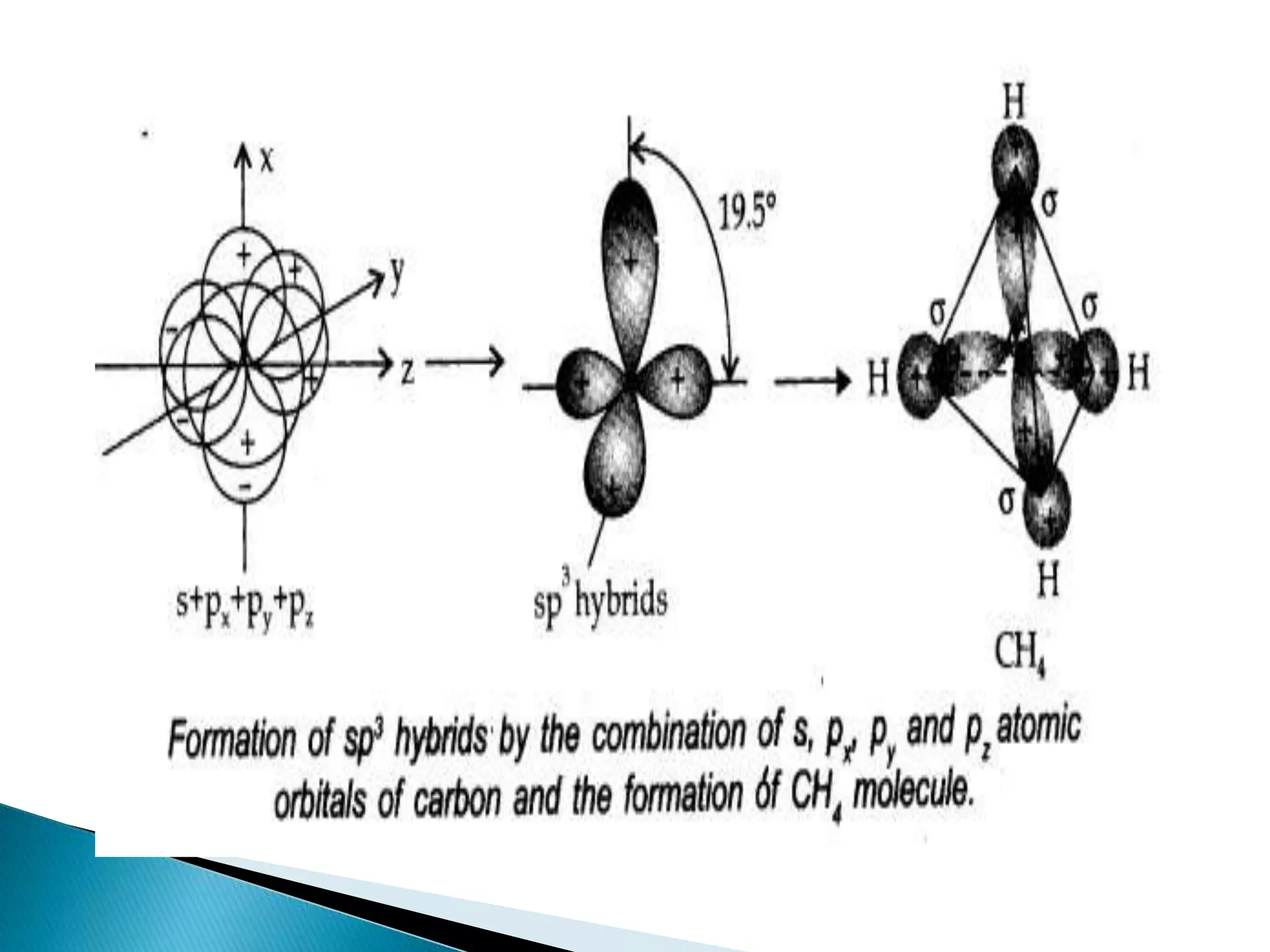
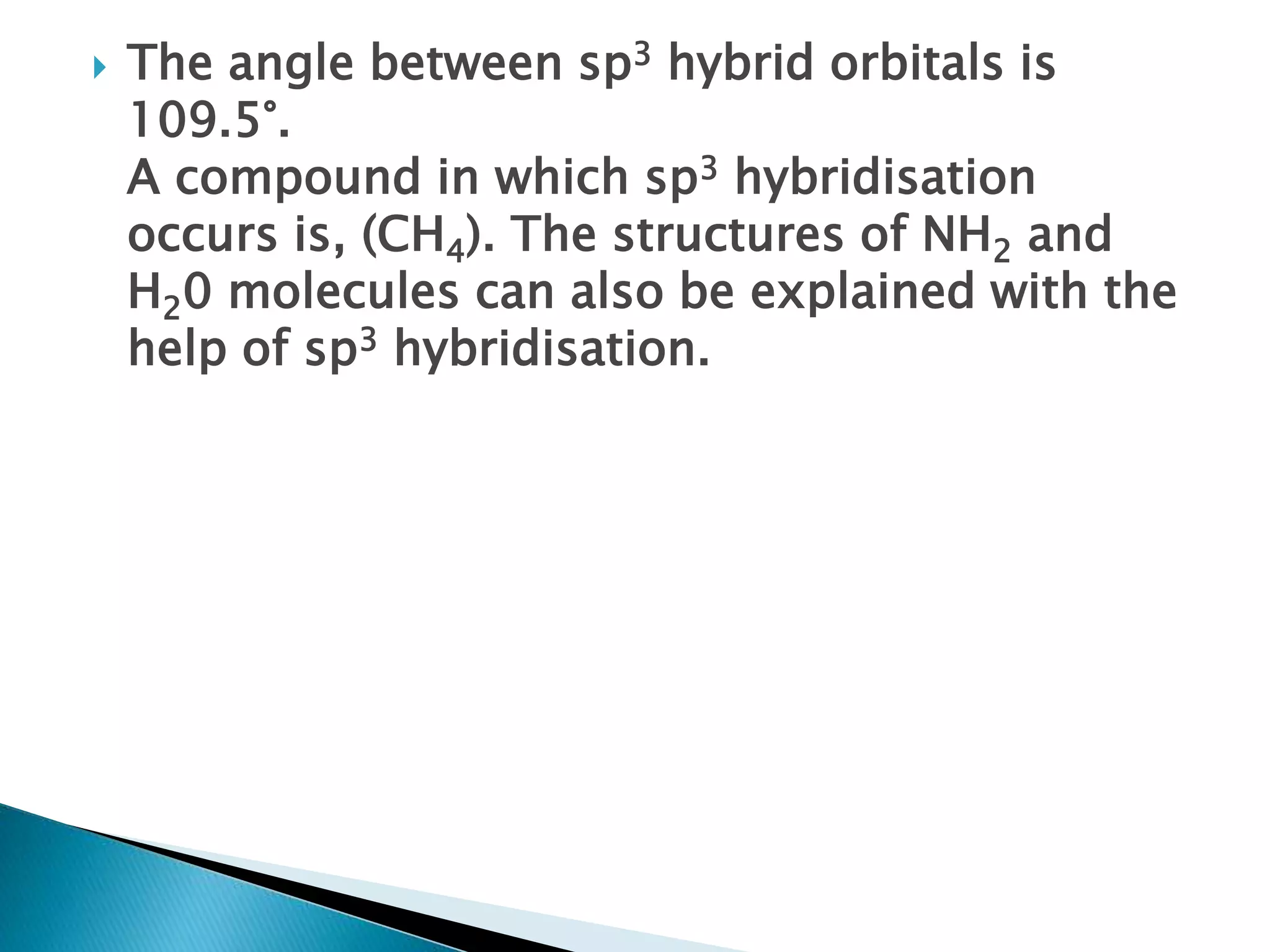





![ Sp2 hybridisation in C2H4
It consists of one sp2-sp2 sigma bond
One pi bond between p orbitals
4 C-H sigma bond sp2-s[108pm]
H-C-H bond angle is 117.6](https://image.slidesharecdn.com/cbms-200921151134/75/chemical-bonding-and-molecular-structure-class-11-115-2048.jpg)